Structure of the Atoms - Revision Notes
CBSE Class 9 Science
Revision Notes
CHAPTER – 4
STRUCTURE OF ATOM
Contribution to the Atomic theory Timeline
- More than 2400 years ago, he named the smallest piece of matter “ATOMOS,” meaning “not to be cut.”
- Atoms were small, hard particles that were all made of the same material but were different shapes and sizes.
- Atoms were infinite in number, always moving and capable of joining together
- Dalton’s Atomic theory:
- First recorded evidence that atoms exists.
- Using his theory, Dalton rationalized the various laws of chemical combination
Dalton's theory was based on the premise that the atoms of different elements could be distinguished by differences in their weights.
Limitations
- The indivisibility of an atom was proved wrong , for, an atom can be further subdivided into protons, neutrons and electrons.
- The atoms of same element are similar in all respects , but isotopes of same element have different mass.
- Dalton's theory was based on the premise that the atoms of different elements could be distinguished by differences in their weights.
- J J Thomson Experiments:
- Discovered electrons in 1897.
- Showed us that the atom can be split into even smaller parts.
His discovery was the first step towards a detailed model of the atom.
- An atom is a uniform sphere of positive charges (due to presence of protons) as well as negative charges (due to presence of electrons).
- Atom as a whole is electrically neutral because the negative and positive charges are equal in magnitude.
- An electron is a negatively charged component of an atom which exists outside the nucleus. Each electron carries one unit of negative charge and has a very small mass as compared with that of a neutron or proton.
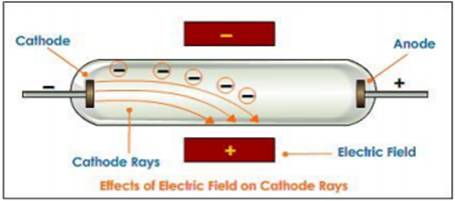
JJ Thomson used cathode ray tubes to demonstrate that the cathode ray responds to both magnetic and electric fields.
Since the ray was attracted to a positive electric plate placed over the cathode ray tube (beam deflected toward the positive plate) he determined that the ray must be composed of negatively charged particles.
He called these negative particles "electrons."
Limitation: Model failed to explain how protons and electrons were arranged in atom so close to each other.
Eugene Goldstein: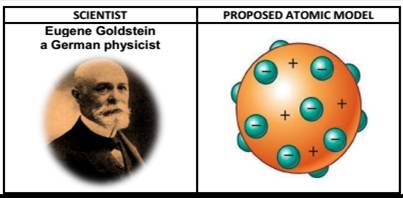
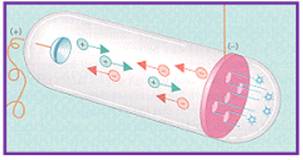
- E. Goldstein in 1886 discovered the presence of new radiations in a gas discharge and called them canal rays. These rays were positively charged radiations which ultimately led to the discovery of another sub-atomic particle.
- Used a Cathode Ray Tube to study "canal rays" which had electrical and magnetic properties opposite of an electron
- Canal Rays: The positively charged radiation produced in the discharge tube at low pressure and high voltage are called canal rays.
Protons
The canal rays have positively charged sub-atomic, particles known as protons (p).
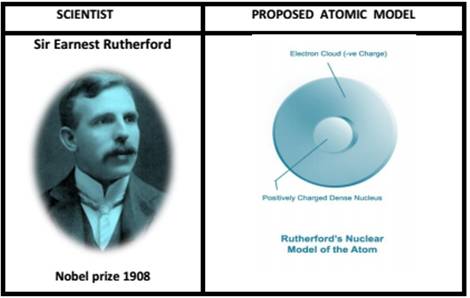
- Rutherford’s Scattering Experiments:
Experiment: Rutherford took a thin gold foil and made alpha particles, positively charged Helium fall on it.
S.No. | OBSERVATION | INFERENCE |
1 | Most of the a-particles passed through the gold foil without getting deflected. | Most of the space inside the atom is empty. |
2 | Very few particles were deflected. | Positive charge of the atom occupies very little space. |
3 | A very few alpha particles, 1 in 100000completely rebound on hitting the goldfoil. | Nucleus of an atom is very small ascompared to the total size. |
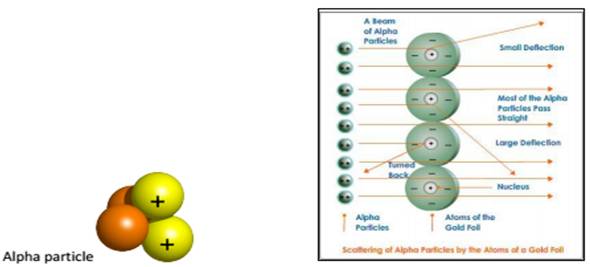
- Limitation: In Rutherford’s atomic model , Nucleus & electrons are held together by electrostatic force of attraction which would lead to the fusion between them. This does not happen in the atom.
Atomic radius ~ 100 pm = 1 x 10 m
Nuclear radius ~ 5 x 10 pm=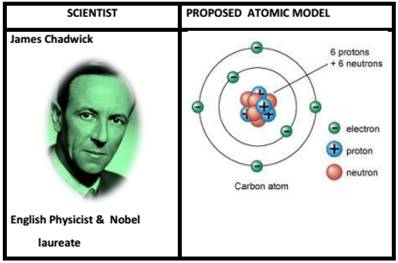
- In 1932, James Chadwick proved that the atomic nucleus contained a neutral particle which had been proposed more than a decade earlier by Ernest Rutherford officially discovered the neutron in 1932,
- Chadwick received the Nobel Prize in 1935.
A neutron is a subatomic particle contained in the atomic nucleus. It has no net electric, unlike the proton's positive electric charge.
4. Sub Atomic Particles:
Name | Symbol | Location in the atom | charge | Relative Mass | Actual Mass (g) |
Electron | E | Around the nucleus | 1– | 1/1840 | |
Proton | P | In the nucleus | 1+ | 1 | |
Neutron | n | In the nucleus | 0 | 1 |
Protons & Neutrons collectively are known as NUCLEONS.
5. Atomic Number & Mass Number:
“Atomic number of an element is defined as the number of unit positive charges on the nucleus (nuclear charge) of the atom of that element or as the number of protons present in the nucleus.”
Atomic number, Z = Number of unit positive charge on the nucleus
= Total number of unit positive charges carried by all protons present in the nucleus.
= Number of protons in the nucleus (p)
= Number of electrons revolving in the orbits (e)
Eg :- Hydrogen – Atomic number = 1 (1 proton)
Helium - Atomic number = 2 (2 protons)
Mass number[ A] : It is defined as the sum of the number of protons & neutrons present in the nucleus of an atom.
Mass Number = Mass of protons + Mass of neutrons
Eg :- Carbon – Mass number = 12 (6 protons + 6 neutrons) Mass = 12u
Aluminium – Mass number = 27 (13 protons + 14 neutrons) Mass = 27u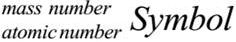
6. Niel Bohr Atomic Model: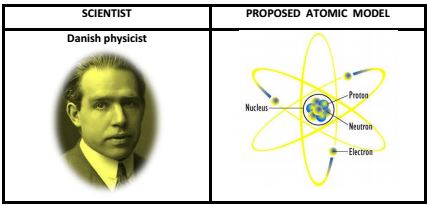
7. Electronic configuration & Valency:
Bohr and Bury Scheme - Important Rules
S.No | where n = shell number | Maximum Capacity |
1 | K Shell 2 x (1) | 2 electrons |
2 | L Shell 2 x (2) | 8 electrons |
3 | M shell 2 x (3) | 18 electrons |
4 | N shell 2 x (4) | 32 electrons |
The outermost shell of an atom cannot accommodate more than 8 electrons, even if it has a capacity to accommodate more electrons. This is a very important rule and is also called the OCTET RULE. The presence of 8 electrons in the outermost shell makes the atom very stable.
8. Isotopes & Isobars
ISOTOPES
ISOTOPES | ISOBARS |
| Chemically same, physically different | Chemically different, physically same |
| Number of electrons is same | Number of electrons is different |
| Cannot be separated by chemical means | Can be separated by chemical means |
