Periodic Classification of Elements - Solutions
CBSE Class 10 Science
NCERT Solutions
Chapter - 5
Periodic Classification of Elements
1. Did Dobereiner’s triads also exist in the columns of Newland’s Octaves? Compare and find out?
Ans. Yes, following triads can be identified:
Li, Na, K
Be, Mg, Ca
The atomic mass of middle atom in the above triads is approximately equal to the average mass of elements on the left and right hand side.
2. What were the limitations of Dobereiner’s classification?
Ans. Dobereiner could identify only three triads from the elements known at that time. Hence, this classification of elements was not found to be useful.
3. What were the limitations of Newland’s law of octaves?
Ans. The limitations of Newlands’ Law of Octaves:
(a) it was found that Law of Octaves was applicable only upto calcium.
After calcium eighth element did not possess properties similar to that of first.
(b) Newlands’ assumed that only 56 elements existed in nature and no more elements would be discovered in future.
(c) To fit the element into his table, Newlands adjusted two elements in the same slot but also put some unlike elements under the same note.
Page No. 85
1. Use Mendeleev’s Periodic Table to predict the formulae for the oxides of the following elements:
K, C, Al, Si, Ba
Ans. K2O, CO2, SiO2, BaO
2. Besides gallium, which other elements have since been discovered that were left by Mendeleev in his Periodic Table? (any two)
Ans. Scandium and Germanium.
3. What were the criteria used by Mendeleev in creating his Periodic table?
Ans. Mendeleev created his Periodic Table on the basis of their fundamental property the atomic mass and also on the similarities of chemical properties. Among chemical properties he concentrated on the compounds formed by elements with oxygen and hydrogen.
4. Why do you think the noble gases are placed in separate group?
Ans. Noble gases are inert elements. Their properties are different from the all other elements. Therefore, the noble gases are placed in a separate group.
Page No. 90
1. How could the Modern Periodic table remove various anomalies of Mendeleev’s Periodic Table?
Ans. Modern Periodic Table settled the placement of isotopes as isotopes have same atomic number.
Position of controversial position of hydrogen is also settled in modern periodic table.
2. Name two elements you would expect to show chemical reactions similar to magnesium. What is the basis for your choice?
Ans. Calcium and Strontium would show chemical reactions similar to magnesium. They have the same number of electrons in the outermost shell.
3. Name:
(a) Three elements that have a single electron in their outermost shells.
(b) Two elements that have two electrons in their outermost shells.
(c) Three elements with filled outermost shells.
Ans. (a) Lithium, sodium and potassium have a single electron in their outermost shells.
(b) Magnesium and calcium have two electrons in their outermost shells.
(c) Helium, neon and argon have filled outermost shells.
4. (a) Lithium, sodium and potassium are all metals that react with water to liberate hydrogen gas. Is there any similarity in the atoms of these elements?
(b) Helium is an un-reactive gas and neon is a gas of extremely low reactivity. What, if anything, do their atoms have in common?
Ans. (a) Lithium, sodium and potassium have same number of electrons in the outermost shell.
(b) Helium and argon have completed outermost shell, 2 electrons in case of helium and 8 electrons in case of argon.
5. In the Modern Periodic Table, which are the metals among the first ten elements?
Ans. Lithium and Beryllium are the metals among the first ten elements in the Modern Periodic Table.
6. By considering their position in the Periodic Table, which one of the following elements would you expect to have maximum metallic characteristic?
Ga, Ge, As, Se, Be
Ans. Metallic character of an element increases down a group and decreases from left to right in period. On this basis Be is expected to have maximum metallic character.
TEXTBOOK EXERCISES
1. Which of the following statements is not correct statement about the trends when going from left to right across the periods of Periodic Table?
(a) The elements become less metallic in nature.
(b) The number of valance electrons increases.
(c) The atoms lose their electrons more easily.
(d) The oxides becomes more acidic.
Ans. (c) The atoms lose their electrons more easily.
2. Element X forms a chloride with the formula XCl2 which is a solid with a high melting point. X would most likely be in the same group of the Periodic Table as
(a) Na
(b) Mg
(c) Al
(d) Si
Ans. (b) Mg
3. Which element has
(a) two cells, both of which are completely filled with electrons?
(b) the electronic configuration 2, 8, 2?
(c) a total of three shells, with four electrons in its valance shell?
(d) twice as many electrons in its second shell as in its first shell?
Ans. (a) Neon
(b) Magnesium
(c) Silicon
(d) Carbon
4. (a) What property do all elements in the same column of the Periodic Table as boron have in common?
(b) What property do all elements in the same column of the Periodic Table as Fluorine have common?
Ans. (a) All the elements in the in the same column as boron have three electrons in the valence shell. That is all the elements are trivalent.
(b) All the elements in the same column as fluorine has one electron in the valance shell, which is all the elements, are monovalent.
5. An atom has electronic configuration 2, 8, 7.
(a) What is the atomic number of this element?
(b)To which of the following elements would it be chemically similar?
(Atomic numbers are given in parentheses.)
N(7), F(9), P(15), Ar(18)
Ans. Chlorine has the electronic configuration 2, 8, 7.
(a) Atomic number of Chlorine element is 17.
(b) F (9)
6. The position of three elements A, B and C in the Periodic Table are shown below:
(a) State whether A is a metal or non-metal.
(b) State whether C is more reactive than A
(c) Will C be larger or smaller in size than B?
(d) Which type of ion, cation or anion, will be formed by element A?
Ans. (a) A is a non-metal.
(b) C is less reactive than A.
(c) C will be smaller than B.
(d) A will form anion.
7. Nitrogen (atomic number 7) and phosphorus (atomic number 15) belong to group 15 of the Periodic Table. Write the electronic configuration of these two elements. Which of these will be more electronegative? Why?
Ans. Nitrogen atomic number 7 has got 7 electrons with electronic configuration 2, 5.
Phosphorus with atomic number 15 has got 15 electrons with electronic configuration 2, 8, 5.
Non-metallic character decreases as we move down the group. Therefore, nitrogen will be more electronegative than phosphorus.
8. How does the electronic configuration of an atom relate to its position in the Modern Periodic Table?
Ans. Group number on an element can be predicted from the number of electrons in the outermost shell.
Period number of an element can be predicted from the number of shells with filled electrons.
Knowing the electronic configuration, we can find the number of electrons in the outermost shell and the number of shells with filled electrons. This can help to relate its position in the Periodic Table.
9. In the modern Periodic Table calcium (atomic number 20) is surrounded by elements with atomic number 12, 19, 21 and 38. Which of these have physical and chemical properties resembling calcium?
Ans. Elements in a group have similar properties. Elements with atomic numbers 12 and 38 lie in the same group as calcium. Therefore, they will have properties resembling calcium.
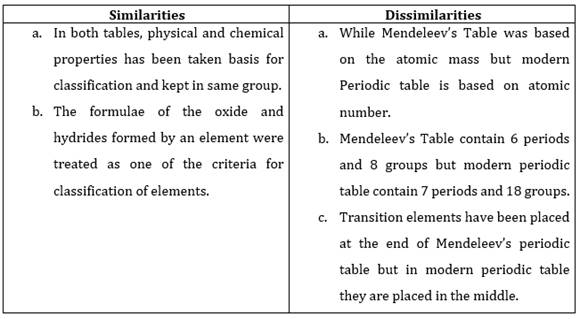
10. Compare and contrast the arrangement of elements in Mendeleev’s Periodic Table and the Modern Periodic Table.
Ans.