Matter in Our Surrounding - Test Papers
CBSE Test Paper 01
Chapter 01 Matter in Our Surrounding
Name the phenomenon which causes one crystal of potassium permanganate to turn a beaker of water purple. (1)
- centrifugation
- filtration
- diffusion
- sedimentation
Name the state of matter that ‘has minimum inter-particle force of attraction’ (1)
- Solid
- Liquid
- gas
- All of these
The inferences drawn by the temperature versus time graph are (1)
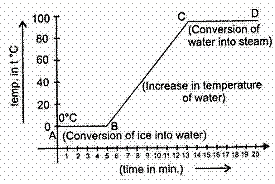
- During the melting, temperature of substance does not change.
- Temperature rises after all amount of ice melts.
- At a specific temperature water starts boiling and temperature remains the same during the conversion of water into steam.
Which statement is correct regarding graph?
- Only (C) is correct
- All (A), (B) and (C) are correct
- Only (B) is correct
- Only (A) is correct
When water boils, its temperature: (1)
- remains the same
- first increases and then decreases
- decreases
- increases
In the determination of boiling point of water correct reading in the thermometer is noted when : (1)
- water starts boiling
- temperature starts rising
- temperature becomes constant
- whole of the water evaporates
What happens when an inflated air balloon is pricked with a pin? Name the property of the gaseous state exhibited by this observation. (1)
Name one property which is show by naphthalene and not by sodium chloride. (1)
Which of the following are matter?Chair, air, love, smell, hate, almonds, thought, cold, cold drink, smell of perfume (1)
Which of the following diffuses faster? Water vapour, wax or, ethyl alcohol. (1)
The room temperature is 25oC. What is the corresponding temperature on the Kelvin scale? (1)
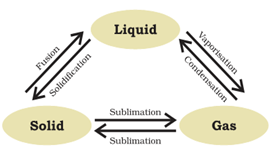
Are the three states of matter inter-convertible? How can they interconnect? (3)
Why are we able to sip hot tea or milk faster from a saucer rather than a cup? (3)
How does evaporation cause cooling? (3)
- Comment on the following statements: (5)
- Evaporation causes cooling.
- Rate of evaporation of an aqueous solution decreases with increase in humidity.
- Sponge though compressible is a solid.
- Ice is solid at 0°C, while water is liquid at room temperature.
- Sugar crystals dissolve faster in hot water than cold water.
- Discuss the various factors which affect the rate of evaporation. Latent heat of evaporation of two liquids A and B is 100 J/kg and 150 J/kg respectively. Which one can produce more cooling effect and why? (5)
CBSE Test Paper 01
Chapter 01 Matter in Our Surrounding
Answers
- diffusion
Explanation: Diffusion is a mass transfer phenomenon that causes the distribution of a chemical species to become more uniform in space as time passes. Potassium permanganate diffusion in water. Beaker containing potassium permanganate (purple) and water, and a clock being used to time how long it takes for the purple colour to spread through the water as the potassium permanganate dissolves. This apparatus is used to demonstrate diffusion in a liquid. Eventually, the random motion of all the potassium permanganate particles results in the purple colour being equally dispersed throughout the water. The process appears slow as the dissolved particles collide with the water molecules and each other, slowing their progress.
- diffusion
- gas
Explanation: The particles of gases are away from each other. So, gas has the minimum inter-particle force of attraction.
Solids have maximum force of attraction
- gas
- All (A), (B) and (C) are correct
Explanation:- During the change of state, given heat is used to change the state. So temperature remains same. AB and CD parts show constant temperature
- BC part represents increase in temperature.
- CD is water starts boiling and temperature remains the same during the conversion of water into steam.
- All (A), (B) and (C) are correct
- remains the same
Explanation: The temperature remains constant during boiling of water even though heat is supplied constantly because all the heat energy provided is used up in changing the state of water from liquid to gaseous water vapour. Therefore all the heat energy provided increases the kinetic energy of the particles and temperature doesn't increase.
- remains the same
- temperature becomes constant
Explanation: Boiling point: the temperature at which the liquid boils and changes into gases state at the atmospheric pressure is called boiling point. In the determination of boiling point of water, correct reading on the thermometer is noted when temperature becomes constant.
- temperature becomes constant
The balloon bursts and the air of balloon gets diffused in air.
- Naphthalene undergoes sublimation upon heating and directly changes into vapours. Sodium chloride (common salt) does not undergo sublimation. It melts on strong heating.
Chair, air, almonds, cold drink, smell of perfume
Water vapour
Kelvin temperature (K) = 273 + 25 = 298 K.
Yes, three states of matter are inter-convertible.
- Solid can be changed into liquid by boiling and liquid can be changed to solid by cooling it i.e. by solidification.
- Liquid can be changed to gas by vaporization by heating it and gas can be changed to liquid by condensation i.e. subjecting it to low temperature.
- Solid can be changed to gaseous form/state by sublimation and gas can be changed to solid by condensation.
Saucer has a bigger surface area as compared to cup. Since evaporation is a surface phenomenon, by using a saucer instead of cup we are increasing the surface area for evaporation to occur. Faster evaporation of particles of tea or milk allows cooling and taking a sip becomes easier.
When a liquid evaporates, the particles of liquid absorb heat from the surroundings to regain the energy lost during evaporation. This absorption of energy from the surroundings
make the surroundings cold.- Evaporation produces cooling as the particles at the surface of the liquid gain energy from the surroundings and change into vapour, thereby producing a cooling effect.
- Air around us cannot hold more than a definite amount of water vapour at a given temperature which is known as humidity. So, if the air is already rich in water vapour, it will not take up more water; therefore, rate of evaporation of water will decrease.
- A sponge has minute holes in which air is trapped. Also the material is not rigid. When we press it, the air is expelled out and we are able to compress.
- Ice is solid at 0°C because it has a definite volume and definite shape due to strong intermolecular forces. Water is liquid at room temperature because it has definite volume and no definite shape due to weak intermolecular forces of attraction.
- Sugar crystals dissolve faster in hot water than cold water because hot water molecules contain more kinetic energy. Due to this, they strike faster on the particles of sugar than cold water molecules. As a result, hot water will dissolve them faster than cold water.
- Factors affecting the rate of evaporation:
- Surface area: The rate of evaporation increases with increase in surface area.
- Temperature: The rate of evaporation increases with increase in temperature.
- Humidity: The rate of evaporation decreases with increase in humidity.
- Wind speed: The rate of evaporation increases with increase in wind speed.
- Nature of the liquid: The volatile compounds evaporate faster than less volatile compounds (liquids).
B will produce more cooling effect because it will absorb more heat from the surroundings for evaporation.
