Matter in Our Surrounding - Solutions
CBSE class IX Science
(Page No. 3)
1. Which of the following are matter?
Chair, air, love, smell, hate, almonds, thought, cold, cold drink, smell of perfume.
Ans. Chair, air, smell, almonds, cold drink and smell of perfume are matter.
2. Give reasons for the following observation:
The smell of hot sizzling food reaches you several metres away, but to get the smell from cold food you have to go close.
Ans. Since hot sizzling food has temperature higher than cold food and at higher temperature diffusion rate (movement) of particles is very fast, due to this the smell of hot sizzling food reaches us from several metres away.
3. A diver is able to cut through water in a swimming pool. Which property of matter does this observation show?
Ans. If diver has ability to cut through water in a swimming pool then it shows that the particles of matter have a kind of force working between them. Because of this force the particles of matter remain together till some external force is applied.
4. What are the characteristics of the particles of matter?
Ans. The characteristics of particles of matter are as follows:
i) Particles of matter have spaces between them.
ii) Particles of matter are in continuous motion.
iii) Particles of matter have an attractive force between them to keep them together.
(Page No. 6)
1. The mass per unit volume of a substance is called density.
(density = mass/volume).
Arrange the following in order of increasing density – air, exhaust from chimneys, honey, water, chalk, cotton and iron.
Ans. Arranging substances in their increasing order of densities:
Air< exhaust from chimneys< cotton< water< honey< chalk< iron.
2. (a) Tabulate the differences in the characteristics of states of matter.
(b) Comment upon the following:
rigidity, compressibility, fluidity, filling a gas container, shape, kinetic energy and density.
Ans. (a)
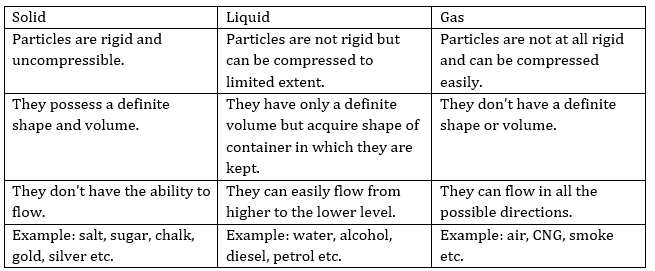
(b) Rigidity→ It is the property of matter to maintain its shape even if external forces work and the solids show this property.
Compressibility → It is the property of matter to allow decrease in volume under high pressure and the gases show this property.
Fluidity → It is the property of a substance to easily flow and allow change in its shape under external forces and this property is exhibited by both liquids and gases.
Filling a gas container → Gases can be compressed easily hence they can be filled within a vessel at high pressure. This property of gases allows their convenient filling into a small container or cylinder and that also in a large volume. It also allows their easy transport from one place to the other e.g. CNG.
Shape→ According to the type of matter shape differs depending upon location of particles like solids have definite shape while liquids acquire the shape of their container and gases as such don’t have any shape.
Kinetic energy → It is the kind of energy present in an object when it is under motion as the particles of that object/matter are continuously moving therefore matter has kinetic energy. However greater is the movement more will be the kinetic energy and vice-a-versa i.e. solid < liquid < gas.
Density → Mass per unit volume of a substance/matter is known as its density i.e. density = mass/volume
3. Give reasons
(a) A gas fills completely the vessel in which it is kept.
(b) A gas exerts pressure on the walls of the container.
(c) A wooden table should be called a solid.
(d) We can easily move our hand in air but to do the same through a solid block of wood we need a karate expert.
Ans.(a) Since the attraction force between particles of a gas is negligible i.e. extremely less hence particles freely move/flow in all possible directions as a result gas fills completely the vessel in which it is kept.
(b) Freely moving particles of gas hit the walls of its container continuously and randomly therefore such random and erratic motion of gas particles exerts pressure on the walls of the container.
(c) The wooden table particles are quite rigid, have a fixed location and also possess a definite shape and volume. Due to all these properties we should call a wooden table a solid substance.
(d) Air is a mixture of gases and since particles of gas are far apart so same is true for air therefore we can easily move our hand in air. But a solid block of wood is hard and rigid that resists any change in location of its particles hence we need a karate expert in case of a solid block of wood.
4. Liquids generally have lower density as compared to solids. But you must have observed that ice floats on water. Find out why.
Ans. When water freezes to form ice,some empty spaces are created. As a result,volume increases for the same mass of water. In other words, mass per unit volume or density of ice is lower than that of water and hence ice floats over water.
(Page No. 9)
1. Convert the following temperature to Celsius scale:
i) 300 K
ii)573 K
Ans. i) 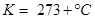
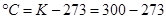
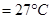
ii) 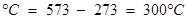
2. What is the physical state of water at:
a. 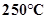
b. 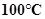
Ans. a) 100C is the boiling point of water. Therefore, at 250C i.e. at a temperature higher than its boiling point is gaseous.
b) At 100C, the boiling point of water, water exists both as a liquid as well as a gas.
3. For any substance, why does the temperature remain constant during the change of state?
Ans. During the change of state the heat or energy provided to particles of matter is utilized in overcoming the forces of attraction of the particles as a result the temperature of substance or matter remains constant during change of state.
4. Suggest a method to liquefy atmospheric gases.
Ans. If we decrease temperature and increase pressure we can liquefy the atmospheric gases.
