Is Matter around Us Pure - Test Papers
CBSE Test Paper 01
Chapter 02 Is matter around us pure
Which of the following settles down when allowed to stand undisturbed doe sometimes? (1)
- Copper sulphate solution
- Blood
- Muddy water
- Solution of egg albumin in water
A mixture of iron filings and sulphur is heated, the colour of the mixture will change (1)
- black to yellow
- yellow to black
- brown to yellow
- black to brown
In the experiment shown a gas is evolved. Four groups of students have recorded their observations on the gas produced as shown in the following table. Choose the correct set of observations. Note that the positive response are shown by
 and negative by
and negative by  signs respectively. (1)
signs respectively. (1)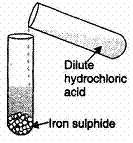
Which of the following solution scatter light? (1)
- None of these
- Both colloidal solution and suspension
- colloidal solution
- suspension
- A Substance can be beaten into sheets and beaten into wires. What will you call it? (1)
- It is both brittle and lustrous
- It is both sonorous and ductile
- It is both Malleable and ductile
- It is both malleable and brittle
Which of the following methods would you use to separate cream from milk? ?(1)
- centrifugation
- filtration
- distillation
- fractional distillation
A shining thick liquid is often used in glass thermometers. Name it. (1)
A saturated solution becomes unsaturated on heating. Why is it so? (1)
How will you justify that rusting if iron is a chemical change? (1)
Define crystallisation. (1)
In what respect does a true solution differ from a colloidal solution? (3)
Describe a method that can be used to separate a mixture of salt and ammonium chloride. (3)
'Sea water can be classified as homogeneous as well as heterogeneous mixture.' Comment. (3)
A compound is regarded as a pure substance while the mixture is not. Give reason. (3)
Fog and cloud are both colloidal in nature. How do they differ? (1)
CBSE Test Paper 01
Chapter 02 Is matter around us pure
Answers
- Muddy water
Explanation: Muddy water will settle down because particles are heavy and settle due to gravity. Setting down of coarse particles under the influence of gravity is called sedimentation. During sedimentation, heavier particles settle down faster than finer particles.
- Muddy water
- yellow to black
Explanation: A mixture of iron filings and sulphur is heated, the colour of the mixture will change yellow to black.
S is black in colour and FeS is black in colour.
Iron + sulphur → Ferrous sulphide
Fe + S → FeS
- yellow to black

Explanation: If we add HCl in FeS it will release H2S Reaction takes place as follows:
FeS + 2HCl → FeCl + H2S
H2S gas turns lead acetate paper black. It is colourless, has smell of rotten eggs, does not catch fire.
- Both colloidal solution and suspension
Explanation: As the particle size of both colloidal & suspension is large they are able to scatter light.
- Both colloidal solution and suspension
- It is both Malleable and ductile
Explanation: The property of metals by which they can be beaten in to thin sheets is called malleability.
The property of metal by which it can be drawn into wires is called ductility.
Gold is most malleable and ductile element.
- It is both Malleable and ductile
- centrifugation
Explanation: In centrifugation by churning the milk at a high speed , the cream collects at the centre and being lighter than milk floats at the top of the mixture .As cream is lighter than milk.
- centrifugation
- The shining liquid is mercury (metal). It is used in glass thermometers as it is the onlyetal which is liquid at room temperature. Besides it does not stick to glass and it has high coefficient of expansion due to which a slight change in temperature can be easily recorded.
- Solubility of a solute (other than gas) increase with the increase in temperature. On heating the liquid develops the capacity of dissolving some more solute to it. That is the saturated solution becomes unsaturated due to increase in the solubility.
- Chemical change can be explained as a change in which a new substance is formed and the process is irreversible. The rust is a brown chemical compound known as hydrated ferric oxide (Fe2O3.xH2O) which is formed when iron reacts with oxygen and water. Formula of rust shows that iron has undergone a chemical change.
- Crystallisation is a process that separates a pure solid in the form of crystals from its solution. It is used to purify solids. E.g. Salt obtained from sea water is purified using crystallisation.
- A true solution is homogeneous mixture whereas a colloidal solution is a heterogeneous mixture.
- A true solution is always clear and transparent whereas a colloidal solution is translucent.
- The diameter of the particles of a solute in a true solution is of the order of 1 nm or less. The size of the colloidal particles is between 1 nm and 100 nm.
- A solute can be recovered from a true solution by evaporation or crystallisation but the particles of a colloidal solution cannot be recovered by evaporation or crystallisation. However, they can be separated through centrifugation.
- Particles of a true solution do not scatter a beam of light whereas particles of a colloidal solution scatter a strong beam of light that is passed through the solution.
- Ammonium chloride exhibits sublimation and changes directly from solid into the gaseous state on heating.
Therefore, a mixture of salt and ammonium chloride can be separated by the process of sublimation.
The following steps would be involved in the separation:-- The mixture of ammonium chloride (NH4Cl) and salt is placed in a china dish. The china dish is placed inside an inverted funnel as shown in the figure.
- The mixture is heated on a low flame. On heating, ammonium chloride sublimes and changes directly into vapours. The vapours of ammonium chloride get condensed on the inner sides of the funnel.
- Salt does not sublime and is left behind in the china dish.
- The fine powder of NH4Cl can be scrapped from the sides of the funnel.
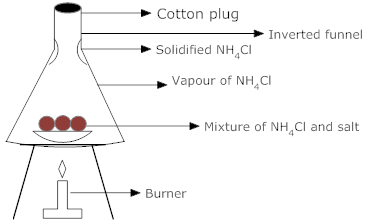
Fig: Separation of NH4Cl and salt by sublimation
- Sea water is a mixture of salts and water which cannot be separated except by evaporation. Therefore, sea water is considered as a homogeneous mixture.
But other than salts and water, sea water also contains mud, decayed plant, etc. So it is considered as a heterogeneous mixture.
Therefore, sea water can be classified as homogeneous as well as heterogeneous mixture - A compound is always a single substance in which two or more elements are combined chemically. A mixture is a combination of elements or compounds or both. Thus, a compound fulfils the definition of a pure substance but not a mixture. Moreover, a compound has a sharp melting or boiling point while a mixture does not have.
- Fog and cloud are the examples in which liquid is the dispersed phase and gas (air) is the dispersion medium. The only difference between them is that clouds are formed in the upper atmosphere while fog gets formed in the region close to earth.



