Is Matter around Us Pure - Solutions
CBSE class IX Science
NCERT Solutions
Chapter 2
Is Matter around Us Pure
(Page No. 15)
1. What is meant by a Pure substance?
Ans: Such substance that has a uniform composition i.e. has particles with identical properties is called pure substance. eg. sugar, salt, water, nitrogen etc.
2. List the points of differences between homogeneous and heterogeneous mixtures.
Ans.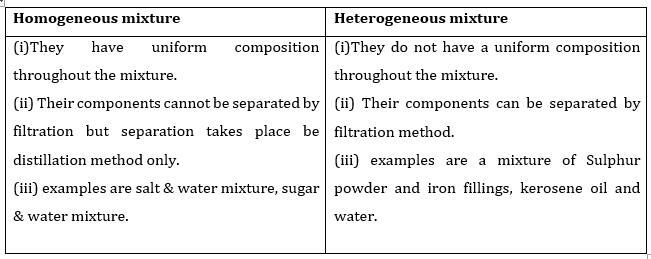
(Page No.18)
1. Differentiate between homogeneous and heterogeneous mixtures with examples.
Ans.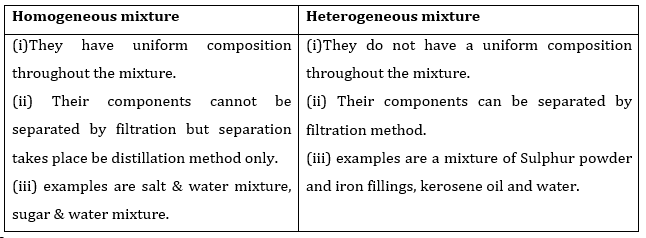
2. How are sol, solution and suspension different from each other?
Ans.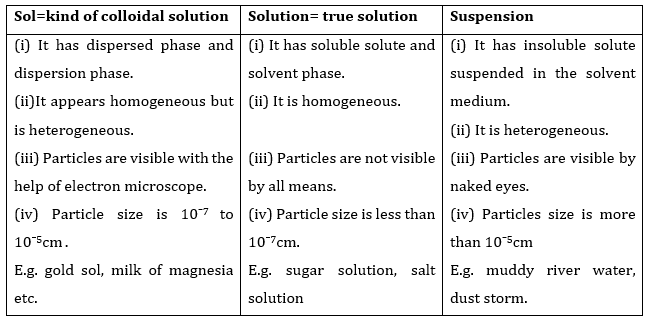
3. To make a saturated solution, 36 g of sodium chloride is dissolved in 100 g of water at 293 K. Find its concentration at this temperature.
Ans.
Mass of solute (sodium chloride) =36g
Mass of solvent (water) =100g
Mass of solution = Mass of Solute + Mass of solvent
= 36g +100g = 136g
(Page No. 24)
1. How will you separate a mixture containing kerosene and petrol (difference in their boiling points is more than 25C), which are miscible with each other?
Ans. We can separate a mixture containing kerosene and petrol by distillation technique since difference in their boiling points is more than 25C. Therefore, they can be easily separated by the technique of simple distillation.
2. Name the technique to separate
(i) butter from curd,
(ii) salt from sea-water,
(iii) camphor from salt.
Ans. (i) centrifugation method.
(ii) evaporation method or crystallisation method
(iii) sublimation method.
3. What type of mixtures are separated by the technique of crystallisation?
Ans. From impure samples of solids, pure solid crystals can be obtained by the method of crystallization for eg to obtain pure sugar from impure sample of the same.
(Page No. 24)
1. Classify the following as chemical or physical changes:
• cutting of trees,
• melting of butter in a pan,
• rusting of almirah,
• boiling of water to form steam,
• passing of electric current through water and the water breaking down into hydrogen and oxygen gases,
• dissolving common salt in water,
• making a fruit salad with raw fruits, and
• burning of paper and wood.
Ans. Cutting of trees = chemical change
Melting of butter in a pan = physical change
Rusting of almirah = chemical change
Boiling of water to form steam = physical change
Passing of electric current through water and the water breaking down into hydrogen and oxygen gases = chemical change
Dissolving common salt in water = physical change
Making a fruit salad with raw fruits = physical change
Burning of paper and wood = chemical change
2. Try segregating the things around you as pure substances or mixtures.
Ans. Distilled water, diamond, graphite, raw rubber are pure substances that can be found around us. In contrast, curd, ice cream, cooking oil , vulcanized rubber are the some of the examples of mixtures.
(Chapter – end)
1. Which separation techniques will you apply for the separation of the following?
(a) Sodium chloride from its solution in water.
(b) Ammonium chloride from a mixture containing sodium chloride and ammonium chloride.
(c) Small pieces of metal in the engine oil of a car.
(d) Different pigments from an extract of flower petals.
(e) Butter from curd.
(f) Oil from water.
(g) Tea leaves from tea.
(h) Iron pins from sand.
(i) Wheat grains from husk.
(j) Fine mud particles suspended in water.
Ans. (a) Evaporation
(b) Sublimation
(c) Filtration
(d) Chromatography
(e) Centrifugation
(f) Separating funnel
(g) Filtration
(h) with the help of a magnet
(i) Blowing air or sieving
(j) using alum
2. Write the steps you would use for making tea. Use the words solution, solvent, solute, dissolve, soluble, insoluble, filtrate and residue.
Ans. Take some amount of solvent (water) in a pan and after heating it add little amount of solute (sugar) to the solvent. Solute will dissolve completely in the solvent forming true solution, then add tea leaves that are insoluble along with another soluble liquid milk. After boiling allow filtration with a sieve so the filtrate you obtain is tea while the residue has tea leaves that are thrown away.
3. Pragya tested the solubility of three different substances at different temperatures and collected the data as given below (results are given in the following table, as grams of substance dissolved in 100 grams of water to form a saturated solution).
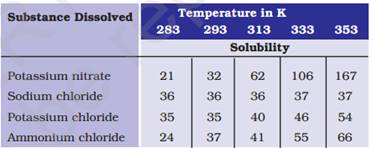
(a) What mass of potassium nitrate would be needed to produce a saturated solution of potassium nitrate in 50 grams of water at 313 K?
(b) Pragya makes a saturated solution of potassium chloride in water at 353 K and leaves the solution to cool at room temperature. What would she observe as the solution cools? Explain.
(c) Find the solubility of each salt at 293 K. Which salt has the highest solubility at this temperature?
(d) What is the effect of change of temperature on the solubility of a salt?
Ans. (a) At 313 K temperature the amount of potassium nitrate required was 62g in 100ml of water so in 50g water we will need to dissolve = 62 X 50/100= 31g potassium nitrate.
(b) When a saturated solution of potassium chloride at 353 K is cooled, the solubility of potassium chloride in water decreases. As a result,the amount of potassium chloride which exceeds its solubility at lower temperature separates out as crystals.
(c) Solubilities are (in 100g of water) 32,36,35,37 respectively for the mentioned salts and the highest solubility is of ammonium chloride at this temperature.
(d) Solubility of salts is directly proportional to the temperature i.e. if temperature increases then solubility will increase and if the temperature decreases solubility will also decrease.
