Carbon and its Compounds - Test Papers
CBSE Test Paper-01
Chapter 04 Carbon and its Compound
The by-product in soap industry is: (1)
- Glycerol
- Isoprene
- Ethylene glycol
- Butane
While cooking, if the bottom of the vessels is getting blackened on the outside, it means that: (1)
- The food is not cooked completely
- The fuel is not burning completely.
- The fuel is burning completely.
- The fuel is wet.
Which of the following is used for antifreeze mixture? (1)
- HCHO
Hardness of water is caused by: (1)
- All of these
Statement A: Acetic acid freezes at 290K.
Statement B: Acetic is also called as glacial acetic acid. (1)- Statement A is true; Statement B is false.
- Statement B is True; Statement A is false.
- Both the statements A and B are true.
- Neither Statement A nor Statement B is true.
Name the chemical reagent which oxidises ethanol to ethanoic acid. (1)
What is the functional group of HCHO? (1)
State the part of soap molecule that attaches itself to dirt when soap is dissolved in water. (1)
An organic compound is a constituent of beer, whisky and some cough syrup. It is produced by the fermentation of sugar. Identify the organic compound. (1)
Explain why soaps are not effective cleansing agents in hard water? (3)
- Write the formula and draw the electron dot structure of carbon tetrachloride.
- What is saponification? Write the reaction involved in this process. (3)
Explain the formation of scum when hard water is treated with soap. (3)
Mention four differences between saturated and unsaturated hydrocarbons. (3)
- What are hydrocarbons? Give examples.
- Give the structural differences between saturated and unsaturated hydrocarbons with two examples each.
- What is functional group? Give examples of four different functional groups. (5)
Describe the addition reaction of carbon compounds with its application. State the function of catalyst in this reaction. How this reaction is different from a substitution reaction? Explain with an example. (5)
CBSE Test Paper-01
Chapter 04 Carbon and its Compound
Answers
- Glycerol
Explanation: Glycerol Is a colourless, sweet, viscous liquid formed as a by-product in soap manufacture. It is used as an emollient and laxative, and for making explosives and antifreeze. The main product is soap.
Fat or Oil + Alkali Soap + Glycerol
- Glycerol
- The fuel is not burning completely.
Explanation: If the bottom of the vessels is getting blackened (due to deposit of soot) on the outside while cooking, it is an indication that the fuel is not burning completely. When the fuel does not burn completely, some carbon particles remain un-oxidised and form soot.
- The fuel is not burning completely.
Explanation: Ethanol () is used for antifreeze mixture. Antifreeze is an additive which lowers the freezing point of a water-based liquid. At room temperature, ethanol is a polar solvent and is used as antifreeze.
- All of these
Explanation: Hardness of water is caused by magnesium and calcium salts. Calcium and magnesium dissolved in water are the two most common minerals that make water hard. Temporary hardness is a type of water hardness caused by the presence of dissolved bicarbonate minerals (calcium bicarbonate and magnesium bicarbonate).
- All of these
- Both the statements A and B are true.
Explanation: Freezing point of ethanoic acid is 17 °C (290 K). When ethanoic acid (acetic acid) is cooled, it freezes to form a colourless, ice-like solid. The solid looks like a glacier and hence pure ethanoic acid is also called glacial ethanoic acid (or glacial acetic acid).
- Both the statements A and B are true.
Ethanol is oxidised by acidifed sodium dichromate in a test-tube reaction, firstly to form ethanal and, with further oxidation, ethanoic acid.
The functional group of HCHO is -CHO.
Hydrophobic end also called as tail, i.e.long hydrocarbon chain moves away from water but attaches to dirt.
Ethanol is a constituent of beer, whisky and some cough syrup. Ethanol is produced by the fermentation of sugar.
It is because detergents form lot of lather even with hard water. Hard water contains Ca2+ and Mg2+ ions which react with soap to form Insoluble salts of calcium and magnesium called scum and soap goes waste. Detergents do not form insoluble compounds with Ca2+ and Mg2+ ions therefore. These are more effective.
- Electronic configuration of carbon, C(6) is
Electronic configuration of chlorine, Cl (17) is
To attain octet configuration, carbon needs 4 electron and chlorine needs 1 electron.
So,carbon forms carbon tetra chloride(CCl4)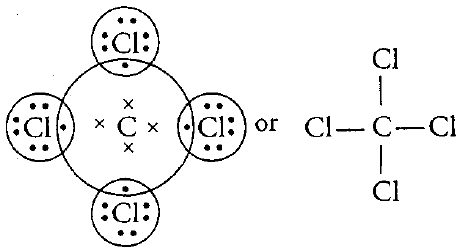
- The reaction of an ester in the presence of base to give sodium salt of carboxylic acid and alcohol is known as saponification and this process is used in the preparation of soap
- Electronic configuration of carbon, C(6) is
Soap does not work properly when the water is hard. A soap is a sodium or potassium salt of long chain fatty acids. Hard water contains salts of calcium and magnesium. When soap is added to hard water, calcium and magnesium ions present in water displace sodium or potassium ions from the soap molecules forming an insoluble substance called scum. A lot of soap is wasted in the process.
Difference between saturated and unsaturated hydrocarbons:
Saturated Hydrocarbons Unsaturated Hydrocarbons Only single bond is present in between carbon-carbon atoms. Double or triple bond is present in between carbon-carbon atoms. Substitution reaction occurs. Addition reaction occurs. They burn with blue flame. They burn with sooty flame. These are Less reactive. These are Highly reactive. - The compounds that are made up of carbon and hydrogen atoms are called hydrocarbons, e.g. methane (CH4), ethane (CH2 = CH2). Ethyne (C2H2), cyclohexane (C6H12), benzene (C6H6)etc.
- In saturated hydrocarbons, all the four valencies of carbon are satisfied by a single covalent bond while in unsaturated hydrocarbons, double or triple bonds are required to satisfy the valencies of carbon, e.g.
- Saturated hydrocarbons
Methane (CH4), Ethane (CH3 — CH3) - Unsaturated hydrocarbons
Ethene (H2C = CH2), Ethyne (HC CH)
- Saturated hydrocarbons
- A functional group is an atom or group of atoms that define the structure (or the properties) of organic compounds. The four examples are:
- -OH Alcohol
- -COOH Carboxylic acid
- -CHO Aldehyde
- -X Halogen
The addition reaction can be seen only with unsaturated carbon compounds. One example of addition reaction is hydrogenation reaction, which is used to obtain ghee from vegetable oil.
e.g.
The rate of reaction increases in the presence of catalyst (Ni or Pt), they adsorb the hydrogen molecule over their surface thus increasing the rate of reaction.
In subsitution reaction, a reagent substitutes on atom or a group of atoms from the reactant instead of addition.
e.g. CH4 + Cl2 Ch3Cl + HCl
