Carbon and its Compounds - Solutions
CBSE Class 10 Science
NCERT Solutions
Chapter - 4
Carbon and Its Compounds
Page No. 61
1. What would be the electron dot structure of carbon dioxide which has the formula of CO2?
Ans.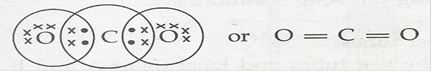
2. What would be the electron dot structure of a molecule of sulphur which is made up of eight atoms of sulphur?
Ans.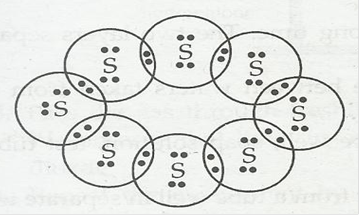
Page No. 68
1. How many structural isomers can you draw for pentane?
Ans. The isomers are as under: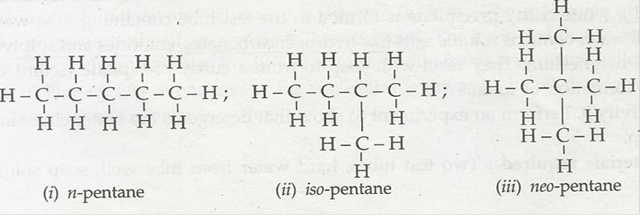
2. What are two properties of carbon which lead to the huge number of carbon compounds we see around us?
Ans. The two properties are:
(a) Catenation- the ability to form bonds with other atoms of carbon.
(b) Tetravalancy of carbon.
3. What will be the formula and electron dot structure for cyclopentane?
Ans. Formula of cyclopentane is C5H10. The electron dot structure cyclcopente is: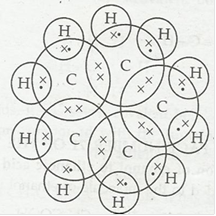
4. Draw the structure for the following compounds:
(i) Ethanoic acid
(ii) Bromopentane
(iii) Butane
(iv) Hexanal
Ans. Structure of compounds are given as under:
(i) Ethanoic acid 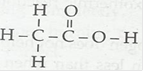
(ii) Bromopentane 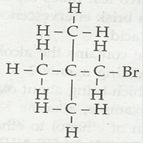
(iii) Butanone 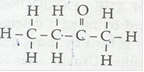
(iv) Hexanal 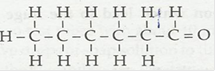
5. How would you name the following compounds?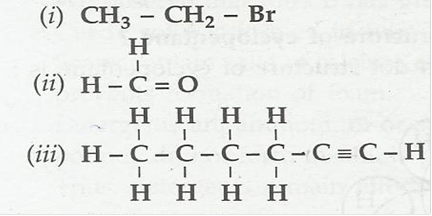
Ans. (i) Bromomethane
(ii) Methanal
(iii) Hexyne
Page No. 76
1. Would you be able to check if water is hard by using a detergent?
Ans. No, we would be able to check if water is hard by using a detergent because detergent form foam with both hard and soft water.
2. People use a variety of methods to wash clothes. Usually after adding the soap, they beat the clothes on stone, or beat it with a paddle, scrub with a brush or the mixture is agitated in a washing machine. Why is agitation necessary to get clean clothes?
Ans. Agitation is necessary to obtain complete mecells formation and the emulsion of oil in water so that the whole of dirt is removed on rinsing with water.
1. Ethane, with the molecular formula C2H6 has
(a) 6 covalent bonds
(b) 7 covalent bonds
(c) 8 covalent bonds
(d) 9 covalent bonds
Ans. (b) 7 covalent bonds
2. Butanone is a four carbon compound with the functional group
(a) carboxylic acid
(b) aldehyde
(c) ketone
(d) alcohol
Ans. (c) Ketone
3. While cooking, if the bottom of the vessels is getting blackened on the outside, it means that
(a) the fuel is not cooked completely.
(b) the fuel is not burning completely.
(c) the fuel is wet.
(d) the is burning completely.
Ans. (b) the fuel is not burning completely.
4. Explain the nature of the covalent bond using the bond formation in CH3Cl.
Ans. Covalent bond is formed by sharing of electrons between two atoms. It is non-ionic in nature.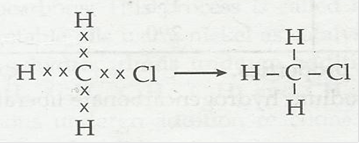
5. Draw the electron dot structure for
(a) Ethanoic acid
(b) H2S
(c) Propanone
(d) F2
Ans. (a) Ethanoic acid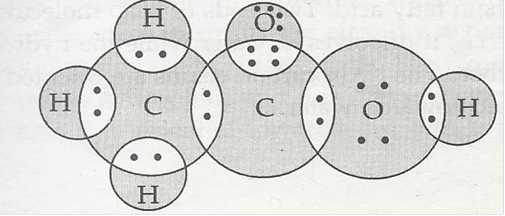
(b) H2S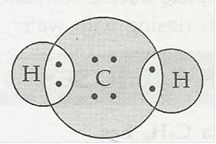
(c) Propanone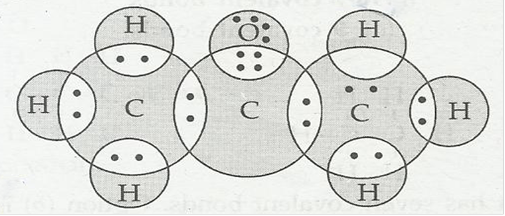
(d) F2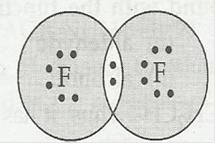
6. What is a homologous series? Explain with an example.
Ans. Series of compounds in which the same functional group substitutes for hydrogen in a carbon chain is called homologues series. The difference between the formulae of any two successive members is –CH2 and difference between the molecular formula is 14u.
7. How can ethanol and Ethanoic acid be differentiated on the basis of their physical and chemical properties?
Ans. On the basis of physical properties:
Melting and boiling points of ethanol is 156 K and 351 K but melting and boiling point of Ethanoic acid is 290K and 391K respectively.
On the chemical properties:
Ethanoic acid reacts with sodium hydrogen carbonate, liberating carbon dioxide while ethanol does not produce carbon dioxide gas.
8. Why does micelle formation take place when soap is added to water? Will a micelle be formed in other solvents such as ethanol also?
Ans. Soap is sodium or potassium salt of long chain fatty acid. Two ends of soap molecules have different properties. The ionic end is hydrophilic. It dissolve in water while the hydrogen chain is hydrophobic, it dissolve in hydrocarbon. The hydrocarbon chains are oriented towards the oil droplet while the ionic ends are oriented towards water.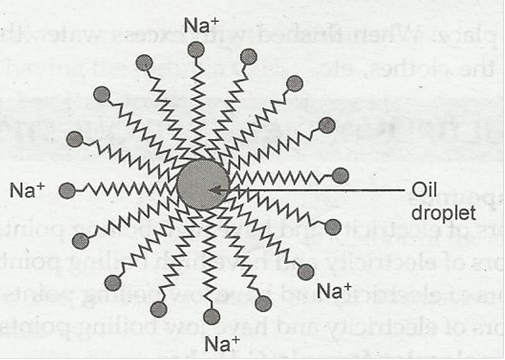
Micelles formation will not take place in ethanol.
9. Why are carbon and its compounds used as fuels for most applications?
Ans. Carbon on combustion gives carbon dioxide and water. This reaction is accompanied by evolution of heat and light. The same is true for compounds of carbon. That is why carbon and its compounds are used as fuel for most applications.
10. Explain the formation of scum when hard water is treated with soap.
Ans. Hard water contains hydrogen carbonates, chlorides and sulphates of calcium and magnesium which reacts with soap to form scum. For example, calcium chloride reacts with soap to form scum.
Sodium stearate + Calcium chloride à sodium chloride + Calcium stearate(scum)
11. What change will you observe if you test soap with litmus paper (red and blue)?
Ans. Soap is sodium or potassium salt of fatty acid. It is obtained by treating oil with caustic soda. Sodium stearate is thus a salt of a weak acid and strong base. Its water solution will be slightly alkaline and will turn red litmus blue.
12. What is hydrogenation? What is its industrial application?
Ans. Unsaturated hydrocarbons add hydrogen in presence of catalysts such as palladium or nickel to give saturated hydrocarbons. This process is called hydrogenation.
It is commercially used for converting vegetable oils to ‘vanaspati’ ghee in presence of nickel as catalyst.
13. Which of the following hydrocarbons undergo addition reactions?
C2H6, C3H8, C3H6, C2H2 and CH4
Ans. C3H6 and C2H2 will undergo addition reactions.
14. Give a test that can be used to differentiate chemically between butter and cooking oil?
Ans. Butter and cooking oil can be differentiated with the help of bromine water test. Cooking oil will decolorize the red colour of bromine water on shaking while butter will not.
15. Explain in mechanism of the cleaning action of soap.
Ans. Soap are sodium or potassium salt of long chain fatty acids. Two ends of molecules of soap behave differently. This ionic end is hydrophilic and it is oriented towards water. The other hydrocarbon end is hydrophobic and it is oriented towards dirt which is oily in nature. A micelle formation around the oily dirt takes place. When flushed with excess of water, the micelle containing the dirt is removed, thus cleaning the clothes, etc.
