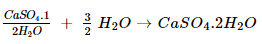Acids Bases and Salts - Solutions
CBSE Class 10 Science
NCERT Solutions
Chapter - 2
Acids, Bases and Salts
1. You have been provided with three test tubes. One of them contains distilled water and the other two contain an acidic solution and a basic solution, respectively. If you are given only red litmus solution, how will you identify the contents of each test tube?
Ans. A few drops of red litmus solution is added to each test tube. Red colour will become light in the test tube containing water. Colour will turn blue in test tube containing basic solution. Red colour will become dark in the test tube containing acidic solution.
Page No. 22
1. Why should curd and sour substance not be kept in brass and copper vessels.
Ans. Brass and copper vessels contain copper and zinc metal that reacts with acids present in curd and sour substance forming soluble salts. These salts are poisonous in nature and make curd unfit for consumption.
2. Which gas is usually liberated when an acid reacts with a metal? Illustrate with an example. How will you test for the presence of this gas?
Ans. Usually hydrogen gas is liberated when an acid reacts with a metal. For example
Zn + 2HCl ZnCl2 + H2
When a burning candle or matchstick is bought near hydrogen gas it burns with pop sound.
3. Metal compound ‘A’ reacts with dilute hydrochloric acid to produce efferenvescence. The gas evolved extinguishes a burning candle. Write a balanced chemical equation for the reaction, if one of the compounds formed is calcium chloride.
Ans. As one of the compounds formed is calcium chloride, metal compound ‘A’ is salt of calcium.
Burning candle is extinguished by carbon dioxide so carbon dioxide gas is produced by reaction of ‘A’ with hydrochloric acid.
Carbon dioxide is produced by action of HCl on carbonate that means ‘A’ is calcium carbonate.
CaCO3 +2HCl CaCl2 + CO2 + H2O
Page No. 25
1. Why do HCl, HNO3 etc. show acidic characters in aqueous solution while solutions of compounds like alcohol and glucose do not show acidic character?
Ans. Compounds like HCl and HNO3 release hydrogen ions in solution, therefore they show acidic character.
While compounds like alcohol and glucose do not release hydrogen ions. Therefore, they do not show acidic properties.
2. Why does an aqueous solution of an acid conduct electricity?
Ans. Electricity is conducted in a solution by ions. Acid release H+ ions in a solution so, it conducts electricity.
3. Why does dry HCl gas not change the colour of the dry litmus paper?
Ans. Colour of litmus paper changes only when it come in contact of H+ ions and H+ ions is produced only when HCl gas comes in contact with water. Therefore dry HCl do not change the colour of dry litmus paper.
4. While diluting an acid, why it is recommended that the acid should be added to water and not water to the acid?
Ans. Addition of water to acid is an exothermic reaction. If we add water to acid lot of heat is produced that may breaks the glass container or sprout to burns the person adding it.
But when acid is added to water with constant stirring, the heat produced is absorbed by water and no harm occurs.
5. How is concentration of hydronium ions (H3O+) affected when a solution of acid is diluted?
Ans. Concentration of hydronium ions decreased when the solution of an acid is diluted.
6. How is concentration of hydroxide ions (OH-) affected when excess base is dissolved in a solution of sodium hydroxide?
Ans. Excess base dissolved in a solution of sodium hydroxide will release more hydroxide (OH-) ions. Therefore, concentration of hydroxide ions (OH-) will increase.
Page No. 28
1. You have two solutions ‘A’ and ‘B’. The pH of solution ‘A’ is 6 and pH of solution ‘B’ is 8. Which solution has more hydrogen ions concentration? Which is acidic and which one is basic?
Ans. A solution having pH less than 7 is acidic and that having pH more than 7 is basic. So, solution ‘A’ is acid and ‘B’ is basic. Naturally ‘A ‘which is acidic has greater concentration of hydrogen ions concentrations.
2. What effect does the concentration of H+ ions have on the nature of the solution?
Ans. Higher the concentration of H+ ions, greater is the acidic nature of the solution.
3. Do basic solutions also have H+ ions? If yes, then why are these basic?
Ans. Acidic and basic solutions both have H+ ions. The difference is that in acids H+ ions concentration is more than OH- ions concentration while in basic solution OH- ions concentration is more than H+ ions concentration.
4. Under what soil condition do you think a farmer would treat the soil of his field with quicklime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate).
Ans. The farmer would treat the soil of his field with quicklime (calcium oxide) or slaked lime (calcium hydroxide) or chalk (calcium carbonate) when field has become acidic to neutralize the effect of acid.
Page No. 33
1. What is the common name of the compound CaOCl2?
Ans. Bleaching powder.
2. Name the substance which on treatment with chlorine yields bleaching powder.
Ans. Slaked lime or calcium hydroxide.
3. Name the sodium compound which is used for softening hard water.
Ans. Sodium carbonate is used for softening hard water.
4. What will happen if a solution of sodium hydrogen carbonate is heated? Give the equation of reaction involved.
Ans. Sodium hydrogen carbonate solution on heating gives sodium carbonate, carbon dioxide and water.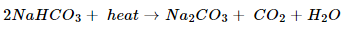
5. Write an equation to show the reaction between plaster of Paris and water.
Ans. The reaction between plaster of Paris and water is as follows: