Acids Bases and Salts - Revision Notes
CBSE Class 10 Science
Acids | Bases |
Sour in taste | Bitter in taste |
Changes blue litmus into red | Changes red litmus into blue |
e.g. Hydrochloric acid HCl | e.g. Sodium hydroxide NaOH |
Sulphuric acid | Potassium hydroxide KOH |
Nitric acid | Calcium hydroxide |
Acetic acid | Ammonium hydroxide |
- Some naturally occurring acids
Vinegar | Acetic Acid |
Orange | Citric Acid |
Lemon | Citric Acid |
Tamarind | Tartaric Acid |
Tomato | Oxalic Acid |
Sour milk (Curd) | Lactic Acid |
Ant and Nettle sting | Methanoic Ac |
- Acid – Base Indicator:Substances which indicate the presence of an acid or base in a solution.
- Litmus solution – It is a natural indicator. It is a purple dye extracted from Lichens. Other examples are Red Cabbage and coloured petals of Petunia and turmeric.
- Olfactory indicators : Show odour changes in acidic or basic media. E.g. onion and clove .
- Acid – Base Indicators
S.No. | Name of the Indicator | Colour Change With Acid | Colour Change with Base |
A. | Blue litmus solution | To red | No change |
B. | Red litmus solution | No change | To blue |
C. | Turmeric | No change | To red |
D. | Methyl orange | To red | To yellow |
E. | Phenolphthalein (colourless) | No change | To pink |
- Dilute Acid : A dilute acid contains a small amount of acid (lower concentration of hydronium ions)and a large amount of water.
- Concentrated Acid :A concentrated acid contains a large amount of acid (higher concentration of hydronium ions)and a small amount of water.
- Chemical Properties of Acids and Bases
- Reaction with metal
- Pop test : When a burning candle is brought near a test tube containing hydrogen gas it burns with a ‘Pop’ sound. This test is conducted for examining the presence of hydrogen gas.
- Base + Metal Salt + Hydrogen
Note– Such reactions are not possible with all the metals.
- Actions of Acids with metal Carbonates and metal bicarbonates
- Lime water Test : On passing the evolved gas through lime water, we find that lime water turns milky.
- the following reaction takes place
- Reaction of acids and bases with each other to give salt and water are called Neutralisation Reactions
e.g.
- Reactions of metal oxides with acids
Note : Appearance of blue green colour of the solution because of formation of .
Metallic oxides are said to be basic in nature because they give salt and water on reacting with acids.Some metallic oxides react with both acids and base and are called AMPHOTER-
IC OXIDES.
- Reaction of Non Metallic Oxide with Base
Non-metallic oxide+ Base Salt + Water
Note : Non Metallic oxides are said to be acidic in nature because on reacting with a base they produce salt and water.
- All acidic solutions conduct electricity because of formation of ions in aq. solution.
- Glowing of bulb indicates that there is a flow of electric current through the solution.
- Acids or bases in a Water Solution
Acids produceions in the presence of water
: Hydronium ion.
ion cannot exist alone. It exists as hydronium ion.
i.e. Base provide ions in the presence of water
- Alkalis
All bases do not dissolve in water. An alkali is a base that dissolves in water.Common alkalis are
- NaOH Sodium hydroxide
- KOH Potassium hydroxide
- Calcium hydroxide
- Ammonium hydroxide
Note : All alkalis are bases but all bases are not alkalis.
- Precaution must be taken while mixing acid or base with water. The acid must always be added to water with constant stirring as it is a highly exothermic reaction.
When an acid or a base is mixed with water they become dilute. This results in the decrease in the concentration of per unit volume in acids and bases respectively,i.e. no. of and reduces.
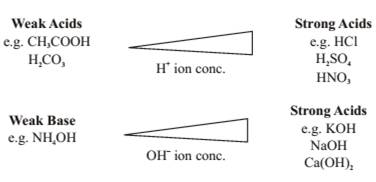
- Strength of an Acid or Base
Strength of acids and bases depends on the no. of and produced respectively.
With the help of a universal indicator we can find the strength of an acid or base as it shows different colours at different concentrations of hydrogen ions in a solution.
A scale for measuring hydrogen ion conc. in a solution called pH scale has been developed.
pH= Potenz in German means power.
This scale measures from 0 (very acidic) to 14 (very alkaline) 7 indicates
Neutral pH (water is neutral).
pH paper : Is a paper which is used for measuring pH
Variation of PH
S. No. PH Colour of the Nature of Solution Conc. Conc.
Value pH Paper
1. | 0 | Dark red | Highly acidic | Very high | Very low |
2. | 4 | Orange or yellow | Acidic | High | Low |
3. | 7 | Green | Neutral | Equal | Equal |
4. | 10 | Bluish green or blue | Alkaline | Low | High |
5. | 14 | Dark blue or violet | highly basic | very low | Very high |
Importance of pH in our daily life
- Importance of pH in our digestive system – Our stomach produces hydrochloric acid. This dilute hydrochloric acid help in digestion of food. In case of indigestion our stomach produces acid in a very large quantity because of which we feel pain and irritation in our stomach (ACIDITY).To get relief from this pain ,antacids are used. These antacids neutralize the excess acid because they are basic in nature and we get relief.
- pH of Acid Rain : When pH of rain water is less than 5.6 it is called acid rain. Flow of acidic rain in water bodies makes them acidic causing a threat to the survival of aquatic life.It also results in damage of structures made with marble like Taj Mahal.
- pH of Soil : Plants require a specific range of pH for their healthy growth. If pH of soil of any particular place is less or more than normal then the farmers add suitable chemicals to it. The addition of these chemicals of presences of excessive damage the nutrients of the soil and decrease its natural fertility.
- Our body functions between the pH range of 7.0 to 7.8. Living organisms can survive only in the narrow range of pH change.
- Tooth decay and pH : Bacteria present in the mouth produces acids by degradation of sugar and food particles remaining in the mouth. Tooth decay begins below the pH 5.6. Using toothpaste which is generally basic, can neutralise the excess acid and prevent tooth decay.
- Bee sting or Nettle sting contains methanoic acid which causes pain and irritation. Using a weak base like baking soda neutralises the acid giving relief.
Salts and their Derivation
S. No. | Name of Salt | Formula | Derived from | Derived from |
1. | Potassium Sulphate | KOH | ||
2. | Sodium Sulphate | NaOH | ||
3. | Sodium Chloride | NaCl | NaOH | HCl |
4. | Ammonium Chloride | HCl |
Note : NaCl andbelong to the family of sodium salts as they have the same radicals. Similarly NaCl and KCl belong to the family of chloride salts.
Neutral Salts : Strong Acid + Strong base
pH value is 7
e.g.NaCl, CaSO
Acidic Salts : Strong Acid + weak base
pH value is less than 7
eq.
Basic Salts : Strong base + weak acid
pH value is more than 7
- NaCl
Sodium chloride is called as common salt. It is derived from sea water.
Rock Salt is mined like coal, is brown coloured and crystalline is shape.
Preparation :
Sodium chloride is obtained by mining the deposits and brine solution is obtained by passing water into the deposits. Hence the salts get dissolved then the solution is pumped out. Evaporation of the sea water is one of the major processes used to obtain salt . The crystals obtained usually consists of impurities such as calcium sulphate, sodium sulphate etc. Pure crystals are obtained by dissolving the salts with little water and filtering the solution.
Uses
- Common salt is an important raw material for many materials of daily use such as.
- Sodium hydroxide
- Washing Soda
- Bleaching Power.
- 2. Used in our food as a preservative and provides flavour to food.
- 3. Used in industries
- Sodium Hydroxide : NaOH, Common Name – caustic soda.
Preparation : Prepared by the method called chlor-alkali process. It is called so because we get chlorine and an alkali (NaOH) in this process.
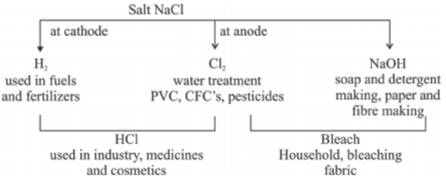
- Calcium oxy chloride -- CaOCl
- The chlorine gas released in brine formation is used to prepare bleach.
Uses
(1) for bleaching cotton and linen in textile industries,wood pulp in paper industry,
(2) Used as disinfectant of water
{3} Used as an oxidising agent.
III. Sodium Hydrogen Carbonate – NaHCO
Common name – Baking Soda. It is mild corrosive base
Preparation :
- Used in baking/cooking
Heating
- produced causes dough to rise and help to make cakes and pastries spongy.
2.Used as ingredients of antacids
3.For preparinf baking soda(baking powder+mild edible acid)
4. Used in soda-acid extinguishers.
Washing Soda
Preparation :Recrystallisation of sodium carbonate.
It is a basic salt used in
- manufacture of Borax.
- `glass,soap and paper industries
- cleansing agent for domestic purposes.
- removing permanent hardness of water.
Water of Crytallization: fixed number of water molecules present in on formula unit of a salt.
Eg:
Plaster of Paris
When Plaster of Paris is mixed with water it changes to gypsum.
: Making toys, decorative material and smoothening surfaces,
plaster for fractured bones.
What you have learnt
Acid-base indicators are dyes or mixtures of dyes which are used to indicate the presence of acids and bases.
- Acidic nature of a substance is due to the formation of (aq) ions in solution. Formation of OH–(aq) ions in solution is responsible for the basic nature of a substance.
- When an acid reacts with a metal, hydrogen gas is evolved and a corresponding salt is formed.
- When a base reacts with a metal, along with the evolution of hydrogen gas a salt is formed which has a negative ion composed of the metal and oxygen.
- When an acid reacts with a metal carbonate or metal hydrogen carbonate, it gives the corresponding salt, carbon dioxide gas and water.
- Acidic and basic solutions in water conduct electricity because they produce hydrogen and hydroxide ions respectively.
- The strength of an acid or an alkali can be tested by using a scale called the pH scale (0-14) which gives the measure of hydrogen ion concentration in a solution.
- A neutral solution has a pH of exactly 7, while an acidic solution has a pH less than 7 and a basic solution a pH more than 7.
- Living beings carry out their metabolic activities within an optimal pH range.
- Mixing concentrated acids or bases with water is a highly exothermic process.
- Acids and bases neutralise each other to form corresponding salts and water.
- Water of crystallisation is the fixed number of water molecules chemically attached to each formula unit of a salt in its crystalline form.
- Salts have various uses in everyday life and in industries.
