States of Matter - Gases and Liquids - Test Papers
CBSE TEST PAPER-01
CLASS - XI CHEMISTRY
(States of Matter: Gases and Liquids)
General Instruction:
- All questions are compulsory.
- Marks are given alongwith their questions.
1. Define Van der waal’s forces. [1]
2. Give an example to show dipole-dipole forces. [1]
3. What type of bond exists between 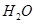 ,HF, NH3, C2H5 OH molecule.? [1]
,HF, NH3, C2H5 OH molecule.? [1]
4. Ice has lower density than water. Give reason. [2]
5. Water has maximum density at 40C. Give reason. [2]
6. Define thermal energy. [2]
7. What are the factors responsible for the strength of hydrogen bonds?[2]
CBSE TEST PAPER-01
CLASS - XI CHEMISTRY (States of Matter: Gases and Liquids)
[ANSWERS]
Ans 1. Attractive intermolecular forces between molecules causing intermolecular bonding is known as Van der waals forces. There are two kinds of Van der Waals forces: weak London Dispersion Forces and stronger dipole-dipole forces.
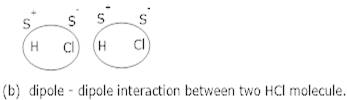
Ans 2. Dipole-dipole forces act between the molecules possessing permanent dipole. Ends of the dipoles possess partial charges which are responsible for the interaction eg. The interaction between two HCl molecules.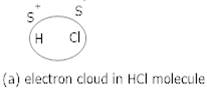
Ans 3. In H2O, HF, NH3, C2H5OH molecule, hydrogen bond exists between hydrogen and the other electronegative atom attached to it.
Ans 4. Hydrogen bonding affect the physical properties of compounds. Ice is H-bonded molecular solid having open type structure with wide holes whereas liquid water has H-bonding having closed type structure that is why ice has lower density than water.
Ans 5. Water has maximum density at 40C because when temperature is increased from 0 to 40C, some of the H-bonds break and molecules come closer and density increases till 40C because of decrease in volume. But, above 40C, the kinetic energy of molecules increases which leads to increase in volume and density decreases.
Ans 6. Thermal energy (Kinetic energy) is the energy of a body arising from motion of its atoms or molecules. It is directly proportional to the temperature of the substance.
Ans 7. Strength of the hydrogen bond is determined by the coulombic interaction between the lone-pair electrons of the electronegative atom of one molecule and the hydrogen atom of other molecule.
