Some Basic Concepts of Chemistry - Revision Notes
CBSE Class 11 Chemistry
- Importance and Scope of Chemistry
- Nature of Matter
- Laws of Chemical Combinations
- Dalton’s Atomic Theory
- Atomic and Molecular Masses
- Stoichiometry and Stoichiometric Calculations
Some Important Points and Terms of the Chapter
1. Anything which has mass and occupies space is called matter.
2. Matters exist in three physical states viz. solid, liquid and gas.
3. In solids, these particles are held very close to each other in an orderly fashion and there is not much freedom of movement. In liquids, the particles are close to each other but they can move around. However, in gases, the particles are far apart as compared to those present in solid or liquid states and their movement is easy and fast.
4. Solids have definite volume and definite shape.
5. Liquids have definite volume but not the definite shape. They take the shape of the container in which they are placed.
6. Gases have neither definite volume nor definite shape. They completely occupy the container in which they are placed.
7. A mixture contains two or more substances present in it (in any ratio) which are called its components.
8. A mixture may be homogeneous or heterogeneous.
9. In a homogeneous mixture, the components completely mix with each other and its composition is uniform throughout. Sugar solution and air are thus, the examples of homogeneous mixtures.
10. In heterogeneous mixtures, the composition is not uniform throughout and sometimes the different components can be observed. For example, the mixtures of salt and sugar, grains and pulses along with some dirt (often stone) pieces, are heterogeneous mixtures..
11. The components of a mixture can be separated by using physical methods such as simple hand picking, filtration, crystallization, distillation etc.
12. Pure substances have characteristics different from the mixtures. They have fixed composition, Copper, silver, gold, water, glucose are some examples of pure substances. Glucose contains carbon, hydrogen and oxygen in a fixed ratio and thus, like all other pure substances has a fixed composition. Also, the constituents of pure substances cannot be separated by simple physical methods.
13. An element consists of only one type of particles. These particles may be atoms or molecules. Sodium, copper, silver, hydrogen, oxygen etc. are some examples of elements. They all contain atoms of one type. However, the atoms of different elements are different in nature. Some elements such as sodium or copper, contain single atoms held together as their constituent particles whereas in some others, two or more atoms combine to give molecules of the element. Thus, hydrogen, nitrogen and oxygen gases consist of molecules in which two atoms combine to give their respective molecules.
14. When two or more atoms of different elements combine, the molecule of a compound is obtained. The examples of some compounds are water, ammonia, carbon dioxide, sugar etc. the atoms of different elements are present in a compound in a fixed and definite ratio and this ratio is characteristic of a particular compound.
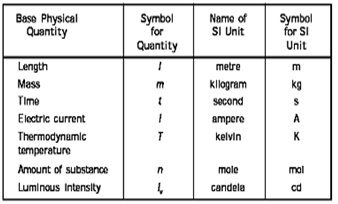
15. The SI system (Systeme International d Unités –abbreviated as SI)) has seven base units and they are listed in
Table 1.1 Base Physical Quantities and their Unites
16. Mass of a substance is the amount of matter present in it while weight is the force exerted by gravity on an object. The mass of a substance is constant whereas its weight may vary from one place to another due to change in gravity.
17. Volume has the units of . So in SI system, volume has units of . A common unit, litre (L) which is not an SI unit, is used for measurement of volume of liquids.
18. Density of a substance is its amount of mass per unit volume SI units of density kg
This unit is quite large and a chemist often expresses density in .
19. There are three common scales to measure temperature Here, K is the SI unit.
20. The Kelvin scale is related to Celsius scale as follows:
21. The °F scale is related to Celsius scale as follows
22. In scientific notation (exponential Notation) any number can be represented in the form where n is an exponent having positive or negative values and N can vary between 1 to 10. Thus, we can write in scientific notation. Note that while writing it, the decimal had to be moved to the left by two places and same is the exponent (2) of 10 in the scientific notation. Similarly, 0.00016 can be written as . Here the decimal has to be moved four places to the right and (– 4) is the exponent in the scientific notation.
23. In Significant figures are meaningful digits which are known with certainty. The uncertainty is indicated by writing the certain digits and the last uncertain digit. Thus, if we write a result as 11.2 mL, we say the 11 is certain and 2 is uncertain and the uncertainty would be 1 in the last digit. Unless otherwise stated, an uncertainty of +1 in the last digit is always understood.
24. There are certain rules for determining the number of significant figures. These are stated below:
a) All non-zero digits are significant. For example in 285 cm, there are three significant figures and in 0.25 mL, there are two significant figures.
b) Zeros preceding to first non-zero digit are not significant. Such zero indicates the position of decimal point. Thus, 0.03 has one significant figure and 0.0052 has two significant figures.
c) Zeros between two non-zero digits are significant. Thus, 2.005 has four significant figures.
d) Zeros at the end or right of a number are significant provided they are on the right side of the decimal point. For example, 0.200 g has three significant figures. But, if otherwise, the terminal zeros are not significant if there is no decimal point. For example, 100 has only one significant figure, but 100. has three significant figures and 100.0 has four significant figures. Such numbers are better represented in scientific notation. We can express the number for one significant figure for two significant figures and for three significant figures.
e) Counting numbers of objects, for example, 2 balls or 20 eggs, have infinite significant figures as these are exact numbers and can be represented by writing infinite number of zeros after placing a decimal i.e.,2 = 2.000000 or 20 = 20.000000
f) In numbers written in scientific notation, all digits are significant e.g has three significant figures, and has four significant figures.
25. Law of Conservation of Mass. Antoine Lavoisier established the Law of Conservation of Mass. It states that matter can neither be created nor destroyed.
26. Law of Definite Proportions states that a given compound always contains exactly the same proportion of elements by weight.
27. Law of Multiple Proportions states that if two elements can combine to form more than one compound, the masses of one element that combine with a fixed mass of the other element, are in the ratio of small whole numbers.
28. Gay Lussac’s Law of Gaseous Volumes: This law was given by Gay Lussac in 1808. He observed that when gases combine or are produced in a chemical reaction they do so in a simple ratio by volume provided all gases are at same temperature and pressure.
29. In 1811, Avogadro proposed that equal volumes of gases at the same temperature and pressure should contain equal number of molecules.
30. In 1808, Dalton published ‘A New System of Chemical Philosophy‘ in which he proposed the following :
a) Matter consists of indivisible atoms.
b) All the atoms of a given element have identical properties including identical mass. Atoms of different elements differ in mass.
c) Compounds are formed when atoms of different elements combine in a fixed ratio.
d) Chemical reactions involve reorganization of atoms. These are neither created nor destroyed in a chemical reaction.
e) Dalton‘s theory could explain the laws of chemical combination.
