Revision Note - Test Papers
CBSE TEST PAPER 01
CLASS XI CHEMISTRY
(Hydrogen)
General Instruction:
- All questions are compulsory.
- Marks are given along with their questions.
1. Which isotope of hydrogen
(i) does not contain neutron?
(ii) is radioactive? [1]
2. Give the electronic configuration of hydrogen. [1]
3. Why does hydrogen occupy unique position in the periodic table? [2]
4. Name the isotopes of hydrogen. [1]
5. Give the main characteristics of isotopes. [2]
6. What is syn-gas? [1]
7. What is coal gasification? [1]
8. Give the laboratory method of preparation of hydrogen. [1]
9. Give the commercial method of preparation of dihydrogen. [1]
10. What is water – gas shift reaction? [1]
CBSE TEST PAPER 01
CLASS XI CHEMISTRY (Hydrogen)
[ANSWERS]
Ans 1. (i) Protium 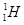 (ii) Tritium
(ii) Tritium 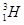
Ans 2. 1s1
Ans 3. Hydrogen has electronic configuration of 1s1.
(i) Some of its properties are similar to alkali metals which have outer electronic configuration of ns1. Hydrogen thus has electronic configuration similar to alkali metals.
(ii) Some of its properties are similar to halogens which have outer electronic configuration of of ns2np5. Hydrogen in this manner resemble halogens as one electron short to the corresponding noble gas configuration- Helium (1s2)
Inspite of the fact that hydrogen, to a certain extent resembles both with alkali metals (ns1) and halogens (ns2 np5), it differs from them as well.Thus, it is unique in its behavior and is therefore, best placed separately in the periodic table.
Ans 4. Hydrogen has three isotopes:
Protium, 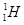 deuterium,
deuterium, 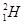 tritium,
tritium, 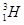
Ans 5. Since, the isotopes have the same electronic configuration, they have almost the same chemical properties. The only difference is in their rates of reactions, mainly due to their different enthalpy of bond dissociation. However, in physical properly of these isotopes differ considerably due to their large mass differences.
Ans 6. The mixture of CO and H2, used for the synthesis of methanol and a number of hydrocarbons, is called synthesis gas or ‘syngas’.
Ans 7. The process of producing syn gas (mixture of CO and H2) from coal is called ‘coal gasification.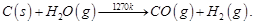
Ans 8. In Lab, Hydrogen is usually prepared by the reaction of granulated zinc with dilute hydrochloric acid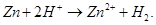
Ans 9.Commercialy, electrolysis of acidified water using platinum electrodes give hydrogen.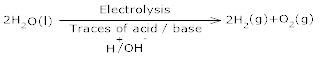
Ans 10. The production of dihyrogen can be increased by reacting carbon monoxide of syn gas mixtures (mixture of CO and H2) with steam in the presence of iron chromate as catalyst.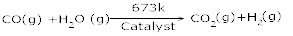
This is called water gas – shift reaction.
Carbon dioxide is removed by scrubbing with sodium arsenite solution.
