Redox Reactions - Solutions
CBSE Class 11 Chemistry
NCERT Solutions
Chapter 8
Redox Reactions
1. Assign oxidation numbers to the underlined elements in each of the following species:
(a) 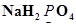
(b) 
(c) 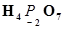
(d) 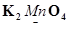
(e) 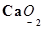
(f)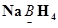
(g) 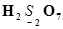
(h) 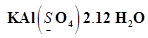
Ans. (a) 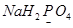
Let the oxidation number of P be x.
We know that,
Oxidation number of Na = +1
Oxidation number of H = +1
Oxidation number of O = -2
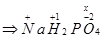
Then, we have
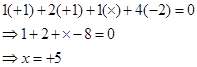
Hence, the oxidation number of P is +5.
(b) 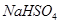
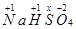
Then, we have: 1(1+1) +1 (+1) +1(x) +4(-2) = 0
= 1+1+ x – 8 = 0
= x = +6
Hence, the oxidation number of S is + 6.
(c) 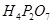
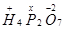
Then, we have: 4(+1) +2(x) +7(-2)
= 4 + 2x – 14 = 0
= 2x = +10
x = +5
Hence, the oxidation number of P is + 5.
(d) 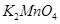
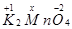
Then, we have: 2(+1) +x +4(-2) = 0
= 2 + x – 8 = 0
= x = +6
Hence, the oxidation number of Mn is + 6.
(e) 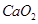
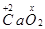
Then, we have: (+2) +2(x) = 0
= 2 + 2x = 0
= x = -1
Hence, the oxidation number of O is -1.
(f) 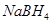
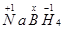
Then, we have
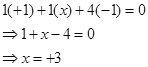
Hence, the oxidation number of B is + 3.
(g) 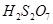
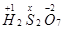
Then, we have
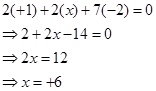
Hence, the oxidation number of S is + 6.
(h) 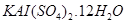
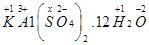
Then, we have
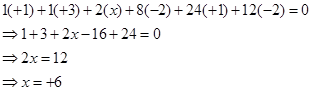
Or,
We can ignore the water molecule as it is a neutral molecule. Then, the sum of the oxidation numbers of all atoms of the water molecule may be taken as zero. Therefore, after ignoring the water molecule, we have
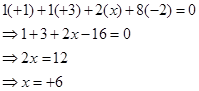
Hence, the oxidation number of S is + 6.
2. What are the oxidation numbers of the underlined elements in each of the following and how do you rationalise your results?
(a) 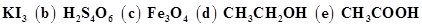
Ans. (a) 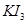
In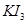 , the oxidation number (O.N.) of K is +1. Hence, the average oxidation number of I is
, the oxidation number (O.N.) of K is +1. Hence, the average oxidation number of I is 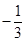 . However, O.N. cannot be fractional. Therefore, we will have to consider the structure of
. However, O.N. cannot be fractional. Therefore, we will have to consider the structure of 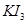 to find the oxidation states.
to find the oxidation states.
In a 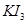 molecule, an atom of iodine forms a coordinate covalent bond with an iodine molecule.
molecule, an atom of iodine forms a coordinate covalent bond with an iodine molecule.
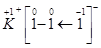
Hence, in a 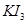 molecule, the O.N. of the two I atoms forming the I2molecule is 0, whereas the O.N. of the I atom forming the coordinate bond is -1.
molecule, the O.N. of the two I atoms forming the I2molecule is 0, whereas the O.N. of the I atom forming the coordinate bond is -1.
(b) 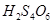
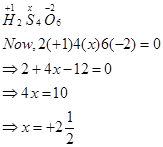
However, O.N. cannot be fractional. Hence, S must be present in different oxidation states in the molecule.
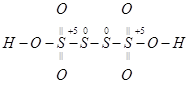
The O.N. of two of the four S atoms is +5 and the O.N. of the other two S atoms is 0.
(c) 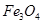
On taking the O.N. of O as -2, the O.N. of Fe is found to be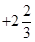 . However, O.N. cannot be fractional.
. However, O.N. cannot be fractional.
Here, one of the three Fe atoms exhibits the O.N. of +2 and the other two Fe atoms exhibit the O.N. of +3.
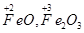
(d) 
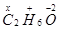
2 (x) + 6 (+1) + 1 (-2) = 0
or, 2x + 4 = 0
or, x = -2
Hence, the O.N. of C is -2.
(e) 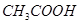
2 (x) + 4 (+1) + 2 (-2) = 0
or, 2x = 0
or, x = 0
However, 0 is average O.N. of C. The two carbon atoms present in this molecule are present in different environments. Hence, they cannot have the same oxidation number. Thus, C exhibits the oxidation states of +2 and -2 in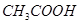 .
.
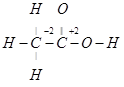
3. Justify that the following reactions are redox reactions:
(a) 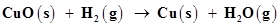
(b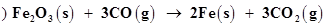
(c)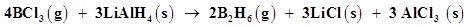
(d) 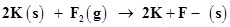
(e) 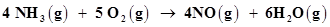
Ans. (a) 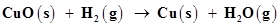
Let us write the oxidation number of each element involved in the given reaction as:
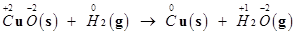
Here, the oxidation number of Cu decreases from +2 in CuO to 0 in Cu i.e., CuO is reduced to Cu. Also, the oxidation number of H increases from 0 in 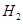 to +1 in
to +1 in 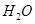 i.e.,
i.e., 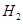 is oxidized to
is oxidized to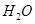 . Hence, this reaction is a redox reaction.
. Hence, this reaction is a redox reaction.
(b) 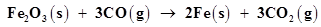
Let us write the oxidation number of each element in the given reaction as:
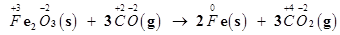
Here, the oxidation number of Fe decreases from +3 in Fe2O3 to 0 in Fe i.e., 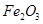 is reduced to Fe. On the other hand, the oxidation number of C increases from +2 in CO to +4 in
is reduced to Fe. On the other hand, the oxidation number of C increases from +2 in CO to +4 in 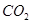 i.e., CO is oxidized to
i.e., CO is oxidized to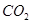 . Hence, the given reaction is a redox reaction.
. Hence, the given reaction is a redox reaction.
(c) 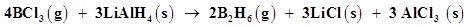
The oxidation number of each element in the given reaction can be represented as:
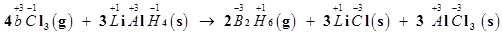
In this reaction, the oxidation number of B decreases from +3 in 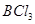 to -3 in
to -3 in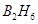 . i.e.,
. i.e., 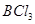 is reduced to
is reduced to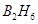 . Also, the oxidation number of H increases from -1 in
. Also, the oxidation number of H increases from -1 in 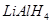 to +1 in
to +1 in 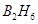 i.e.,
i.e., 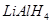 is oxidized to
is oxidized to 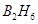 . Hence, the given reaction is a redox reaction.
. Hence, the given reaction is a redox reaction.
(d) 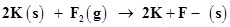
The oxidation number of each element in the given reaction can be represented as:
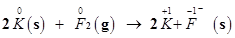
In this reaction, the oxidation number of K increases from 0 in K to +1 in KF i.e., K is oxidized to KF. On the other hand, the oxidation number of F decreases from 0 in 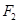 to -1 in KF i.e.,
to -1 in KF i.e., 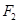 is reduced to KF.
is reduced to KF.
Hence, the above reaction is a redox reaction.
(e) 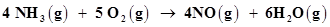
The oxidation number of each element in the given reaction can be represented as:
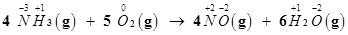
Here, the oxidation number of N increases from -3 in 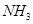 to +2 in NO. On the other hand, the oxidation number of
to +2 in NO. On the other hand, the oxidation number of 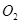 decreases from 0 in
decreases from 0 in 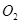 to -2 in NO and
to -2 in NO and 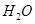 i.e.,
i.e., 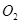 is reduced. Hence, the given reaction is a redox reaction.
is reduced. Hence, the given reaction is a redox reaction.
4. Fluorine reacts with ice and results in the change:
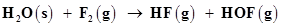
Justify that this reaction is a redox reaction.
Ans. Let us write the oxidation number of each atom involved in the given reaction above its symbol as:
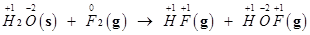
Here, we have observed that the oxidation number of F increases from 0 in 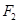 to +1 in HOF. Also, the oxidation number decreases from 0 in
to +1 in HOF. Also, the oxidation number decreases from 0 in 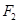 to -1 in HF. Thus, in the above reaction, F is both oxidized and reduced. Hence, the given reaction is a redox reaction.
to -1 in HF. Thus, in the above reaction, F is both oxidized and reduced. Hence, the given reaction is a redox reaction.
5. Calculate the oxidation number of sulphur, chromium and nitrogen in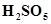 ,
,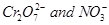 . Suggest structure of these compounds. Count for the fallacy.
. Suggest structure of these compounds. Count for the fallacy.
Ans. (i) 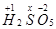
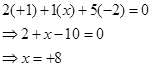
However, the O.N. of S cannot be +8. S has six valence electrons. Therefore, the O.N. of S cannot be more than +6.
The structure of 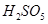 is shown as follows:
is shown as follows:
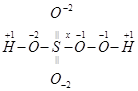
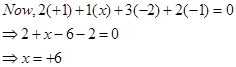
Therefore, the O.N. of S is +6.
(ii) 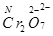
Here, there is no fallacy about the O.N. of Cr in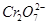 .
.
The structure of 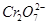 is shown as follows:
is shown as follows:
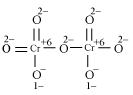
Here, each of the two Cr atoms exhibits the O.N. of +6.
(iii)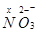
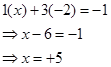
Here, there is no fallacy about the O.N. of N in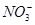 .
.
The structure of 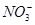 is shown as follows:
is shown as follows:
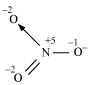
The N atom exhibits the O.N. of +5.
6. Write the formulae for the following compounds:
(a) Mercury(II) chloride
(b) Nickel(II) sulphate
(c) Tin(IV) oxide
(d) Thallium(I) sulphate
(e) Iron(III) sulphate
(f) Chromium(III) oxide 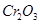
Ans. (a) Mercury (II) chloride: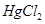
(b) Nickel (II) sulphate: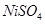
(c) Tin (IV) oxide: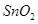
(d) Thallium (I) sulphate: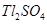
(e) Iron (III) sulphate: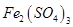
(f) Chromium (III) oxide: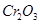
7. Suggest a list of the substances where carbon can exhibit oxidation states from -4 to +4 and nitrogen from -3 to +5.
Ans. The substances where carbon can exhibit oxidation states from -4 to +4 are listed in the following table.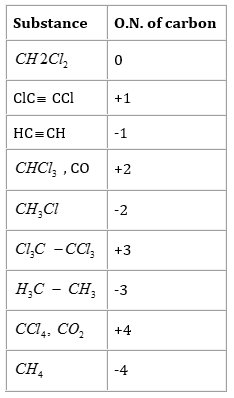
The substances where nitrogen can exhibit oxidation states from -3 to +5 are listed in the following table.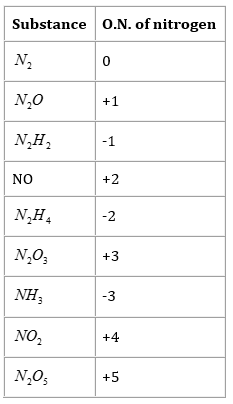
8. While sulphur dioxide and hydrogen peroxide can act as oxidising as well as reducing agents in their reactions, ozone and nitric acid act only as oxidants. Why?
Ans. In sulphur dioxide (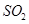 ), the oxidation number (O.N.) of S is +4 and the range of the O.N. that S can have is from +6 to -2.
), the oxidation number (O.N.) of S is +4 and the range of the O.N. that S can have is from +6 to -2.
Therefore, 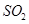 can act as an oxidising as well as a reducing agent.
can act as an oxidising as well as a reducing agent.
In hydrogen peroxide (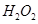 ), the O.N. of O is -1 and the range of the O.N. that O can have is from 0 to -2. O can sometimes also attain the oxidation numbers +1 and +2. Hence,
), the O.N. of O is -1 and the range of the O.N. that O can have is from 0 to -2. O can sometimes also attain the oxidation numbers +1 and +2. Hence, 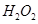 can act as an oxidising as well as a reducing agent.
can act as an oxidising as well as a reducing agent.
In ozone (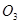 ), the O.N. of O is zero and the range of the O.N. that O can have is from 0 to -2. Therefore, the O.N. of O can only decrease in this case. Hence,
), the O.N. of O is zero and the range of the O.N. that O can have is from 0 to -2. Therefore, the O.N. of O can only decrease in this case. Hence, 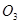 acts only as an oxidant.
acts only as an oxidant.
In nitric acid (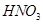 ), the O.N. of N is +5 and the range of the O.N. that N can have is from +5 to -3. Therefore, the O.N. of N can only decrease in this case. Hence,
), the O.N. of N is +5 and the range of the O.N. that N can have is from +5 to -3. Therefore, the O.N. of N can only decrease in this case. Hence, 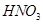 acts only as an oxidant.
acts only as an oxidant.
9. Consider the reactions:
(a) 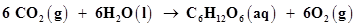
(b) 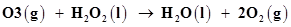
Why it is more appropriate to write these reactions as:
(a) 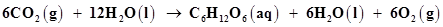
(b) 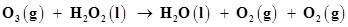
Also suggest a technique to investigate the path of the above (a) and (b) redox reactions.
Ans. (a)The process of photosynthesis involves two steps.
Step 1:
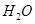 decomposes to give
decomposes to give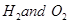 .
.
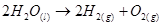
Step 2:
The 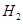 produced in step 1reduces
produced in step 1reduces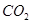 , thereby producing glucose
, thereby producing glucose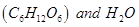 .
.
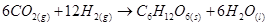
Now, the net reaction of the process is given as:
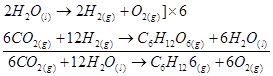
It is more appropriate to write the reaction as given above because water molecules are also produced in the process of photosynthesis.
The path of this reaction can be investigated by using radioactive 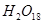 in place of
in place of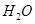 .
.
(b)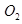 is produced from each of the two reactants
is produced from each of the two reactants 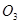 and
and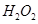 . For this reason,
. For this reason, 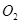 is written twice.
is written twice.
The given reaction involves two steps. First, 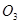 decomposes to form
decomposes to form 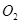 and O. In the second step,
and O. In the second step,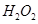 reacts with the O produced in the first step, thereby producing
reacts with the O produced in the first step, thereby producing 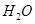 and
and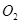 .
.
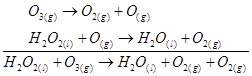
The path of this reaction can be investigated by using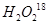 or
or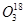 .
.
10. The compound 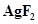 is an unstable compound. However, if formed, the compound acts as a very strong oxidizing agent. Why?
is an unstable compound. However, if formed, the compound acts as a very strong oxidizing agent. Why?
Ans. The oxidation state of Ag in 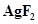 is +2. But, +2 is an unstable oxidation state of Ag. Therefore, whenever
is +2. But, +2 is an unstable oxidation state of Ag. Therefore, whenever 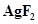 is formed, silver readily accepts an electron to form Ag+. This helps to bring the oxidation state of Ag down from +2 to a more stable state of +1. As a result,
is formed, silver readily accepts an electron to form Ag+. This helps to bring the oxidation state of Ag down from +2 to a more stable state of +1. As a result, 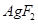 acts as a very strong oxidizing agent.
acts as a very strong oxidizing agent.
