Materials Metals and Non Metals - Exemplar Solutions 4
CBSE Class 8 Science
NCERT Exemplar Solution
CHAPTER 4
Materials: Metals and Non-Metals
NCERT Exemplar Solution
CHAPTER 4
Materials: Metals and Non-Metals
LONG ANSWER QUESTIONS
28. Some of the following statements are incorrect. Find the incorrect statements and correct them.
(a) The property of metals by virtue of which they can be drawn into wires is called ductility.
(b) Metals are good conductor of electricity but poor conductor of heat.
(c) Articles made of metals produce ringing sound when struck hard.
(d) Oxides of non-metals and metals are acidic in nature.
(e) A less reactive metal replaces a more reactive metal from its salt solution in water.
(b) Metals are good conductor of electricity but poor conductor of heat.
(c) Articles made of metals produce ringing sound when struck hard.
(d) Oxides of non-metals and metals are acidic in nature.
(e) A less reactive metal replaces a more reactive metal from its salt solution in water.
Ans. Statements (b), (d) and (e) are not correct.
(b) Metals are good conductor of electricity and also good conductor of heat.
(d) Oxides of non-metals are acidic in nature while oxides of metals are basic in nature.
(e) A more reactive metal replaces a less reactive metal from its salt solution in water.
(b) Metals are good conductor of electricity and also good conductor of heat.
(d) Oxides of non-metals are acidic in nature while oxides of metals are basic in nature.
(e) A more reactive metal replaces a less reactive metal from its salt solution in water.
29. Iron is more reactive than copper. Can you write an activity to show this?
Ans. The activity based on the fact that when an iron nail is put in a beaker containing copper sulphate solution, iron replaces copper from the solution, since it is more reactive. Copper metal and iron sulphate are the products which are obtained as a result of the chemical reaction.
The basic reactivity series of metals is:
potassium, sodium, lithium, calcium, magnesium, aluminium, zinc, iron, tin, lead, copper, silver, gold, platinum
potassium, sodium, lithium, calcium, magnesium, aluminium, zinc, iron, tin, lead, copper, silver, gold, platinum
30. Fill in the blanks to complete the following paragraph.
The name of the product formed in the reaction of sulphur and _______ is sulphur dioxide gas. When sulphur dioxide is dissolved in ________, sulphurous acid is formed. The sulphurous acid turns _______ litmus paper to _ ________. Generally, oxides of _______ are acidic in nature.
After completing the paragraph write two questions which you can raise on the basis of this information.
Ans. Oxygen, water, blue, red, non-metals.
Questions maybe
(i) Which gas is formed when sulphur reacts with oxygen?
(ii) What is the nature of oxides of non-metals?
31. Find out the names of three metals and three non-metals from the box given as Fig 4.2.
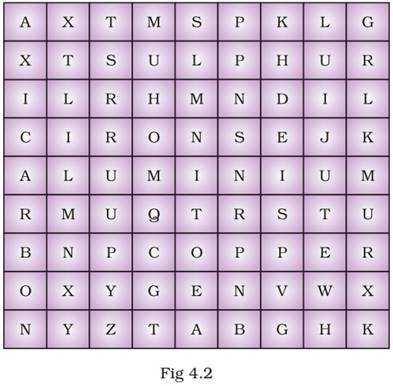
Ans.
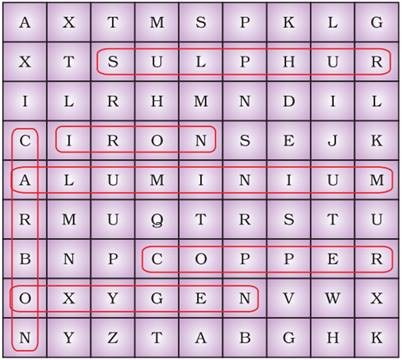
Ans.
Metals
|
Non-metals
|
Iron
|
Carbon
|
Copper
|
Oxygen
|
Aluminium
|
Sulphur
|
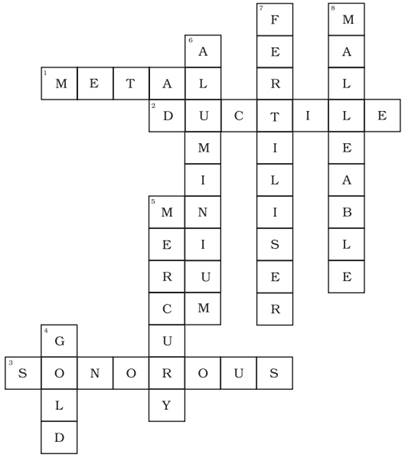
32. Complete the crossword given in Fig. 4.3 with the help of the clues.
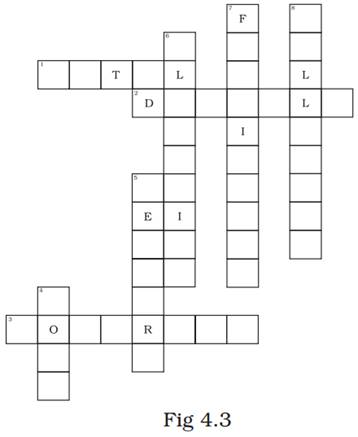
Across
1. Which is generally hard, ductile, malleable and sonorous.
2. A metal is called so it can be drawn into wires.
3. Metal bells are used because of this property.
Down
4. A metal generally used for making jewellery.
5. A metal which is liquid at room temperature.
6. A metal which reacts with acid as well as base to form hydrogen gas.
7. Substances used to enhance the growth of plants.
8. Property by virtue of which metals can be beaten into thin sheets.
Ans.