Hydrocarbons - Test Papers
CBSE TEST PAPER 01
CLASS XI CHEMISTRY (Hydrocarbons)
General Instruction:
- All questions are compulsory.
- Marks are given alongwith their questions.
1. Classify the hydrocarbons according to the carbon – carbon bond. [1]
2. What are cycloalkanes? [1]
3. The boiling point of hydrocarbons decreases with increase in branching. Give reason. [2]
4. Unsaturated compounds undergo addition reactions. Why? [2]
5. Why does carbon have a larger tendency of catenation than silicon although they have same number of electrons? [1]
6. To which category of compounds does cyclohexane belong? [1]
7. Draw the structure of the following compounds all showing C and H atoms.
(a) 2-methyl -3-iso propyl heptanes
(b) Dicyclopropyl methane. [2]
8. Draw all the possible structural isomers with the molecular formula C6H14, Name them. [2.5]
9. Write IVPAC names of the following
CH3 (CH2)4 CH (CH2)3 CH3 -CH2 – CH (CH3)2. [1]
CBSE TEST PAPER 01
CLASS XI CHEMISTRY (Hydrocarbons)
[ANSWERS]
Ans 01. Hydrocarbons are categorized into three categories according to the carbon – carbon bond that exists between then-
(a) saturated hydrocarbon (b) Unsaturated hydrocarbon (c) Aromatic hydrocarbon.
Ans 02. When carbon atoms form a closed chain or a ring, they are termed as cycloalkanes.
Ans 03. Branching result into a more compact (nearly spherical) structure. This reduces the effective surface area and hence the strength of the Vander wall’s forces, thereby leading to a decrease in the boiling point.
Ans 04. Unsaturated hydrocarbon compounds contain carbon – carbon double or triple bonds. The π-bond is multiple bond and is unstable and therefore addition takes place across the multiple bonds.
Ans 05. It is due to the smaller size of C than Si which catenates with stronger C-C bond (335 KJ mol-1) than Si-Si bond (225.7 KJ mol-1).
Ans 06. Saturated alicyclic hydrocarbons.
Ans 07. (a)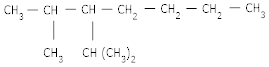
(b)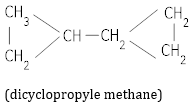
Ans 08. (i)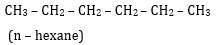
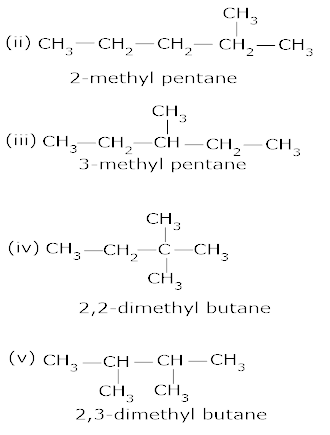
Ans 09. 5-(2 – Methyl propyl) – decane.
