Hydrocarbons - Solutions
CBSE Class 11 Chemistry
NCERT Solutions
Chapter 13
HYDROCARBONS
1. How do you account for the formation of ethane during chlorination of methane?
Ans. Chlorination of methane proceeds via a free radical chain mechanism. The whole reaction takes place in the given three steps.
Step 1: Initiation:
The reaction begins with the homolytic cleavage of Cl- Cl bond as: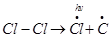
Chlorine free radicals
Step 2: Propagation:
In the second step, chlorine free radicals attack methane molecules and break down the C-H bond to generate methyl radicals as: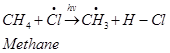
These methyl radicals react with other chlorine free radicals to form methyl chloride along with the liberation of a chlorine free radical.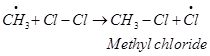
Hence, methyl free radicals and chlorine free radicals set up a chain reaction. While HCl and 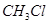 are the major products formed, other higher halogenated compounds are also formed as:
are the major products formed, other higher halogenated compounds are also formed as: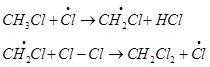
Step 3: Termination:
Formation of ethane is a result of the termination of chain reactions taking place as a result of the consumption of reactants as: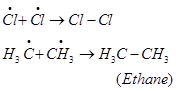
Hence, by this process, ethane is obtained as a by-product of chlorination of methane.
2. Write IUPAC names of the following compounds:
a. 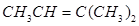
b. 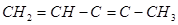
c. 
d. 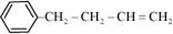
e. 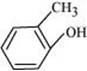
f. 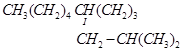
g. 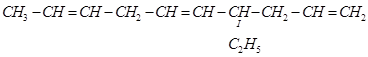
Ans. (a) 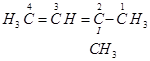
IUPAC name: 2-Methylbut-2-ene
(b)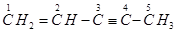
IUPAC name: Pen-1-ene-3-yne
(c)  can be written as:
can be written as: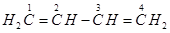
IUPAC name: 1, 3-Butadiene or Buta-1,3-diene
(d) 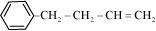
IUPAC name: 4-Phenyl but-1-ene
(e) 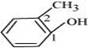
IUPAC name: 2-Methyl phenol
(f) 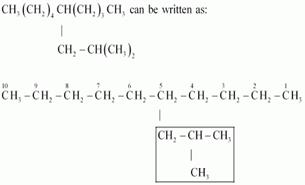
IUPAC name: 5-(2-Methylpropyl)-decane
(g) 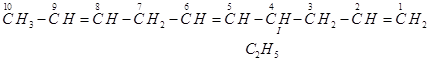
IUPAC name: 4-Ethyldeca-1, 5, 8-triene
3. For the following compounds, write structural formulas and IUPAC names for all possible isomers having the number of double or triple bond as indicated:
(a) 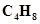 (one double bond)
(one double bond)
(b) 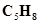 (one triple bond)
(one triple bond)
Ans. (a) The following structural isomers are possible for 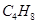 with one double bond:
with one double bond: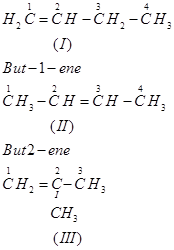
The IUPAC name of
Compound (I) is But-1-ene,
Compound (II) is But-2-ene, and
Compound (III) is 2-Methylprop-1-ene.
(b) The following structural isomers are possible for 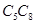 with one triple bond:
with one triple bond: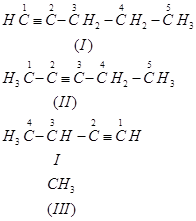
The IUPAC name of
Compound (I) is Pent-1-yne,
Compound (II) is Pent-2-yne, and
Compound (III) is 3-Methylbut-1-yne.
4. Write IUPAC names of the products obtained by the ozonolysis of the following compounds:
(i) Pent-2-ene
(ii) 3,4-Dimethyl-hept-3-ene
(iii) 2-Ethylbut-1-ene
(iv) 1-Phenylbut-1-ene
Ans. (i) Pent-2-ene undergoes ozonolysis as: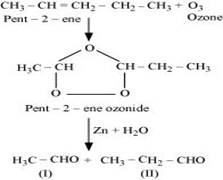
The IUPAC name of Product (I) is ethanal and Product (II)is propanal.
(ii) 3, 4-Dimethylhept-3-ene undergoes ozonolysis as: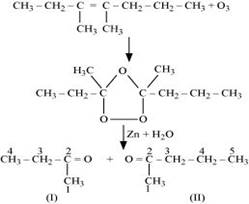
The IUPAC name of Product (I)is butan-2-one and Product (II)is Pentan-2-one.
(iii) 2-Ethylbut-1-ene undergoes ozonolysis as: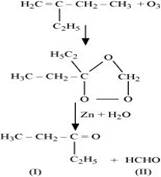
The IUPAC name of Product (I)is pentan-3-one and Product (II)is methanal.
(iv) 1-Phenylbut-1-ene undergoes ozonolysis as: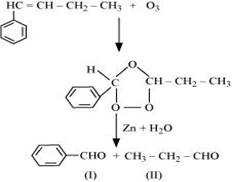
The IUPAC name of Product (I)is benzaldehyde and Product (II)is propanal.
5. An alkene 'A' on ozonolysis gives a mixture of ethanal and pentan-3-one. Write structure and IUPAC name of 'A'.
Ans. 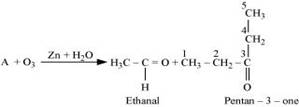
During ozonolysis, an ozonide having a cyclic structure is formed as an intermediate which undergoes cleavage to give the final products. Ethanal and pentan-3-one are obtained from the intermediate ozonide. Hence, the expected structure of the ozonide is: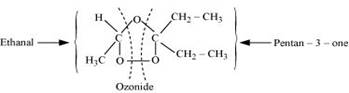
This ozonide is formed as an addition of ozone to ‘A'. The desired structure of ‘A' can be obtained by the removal of ozone from the ozonide. Hence, the structural formula of ‘A' is: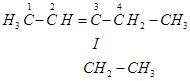
The IUPAC name of ‘A' is 3-Ethylpent-2-ene.
6. An alkene 'A' contains three C - C, eight C - H 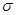 bonds and one C - C
bonds and one C - C 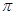 bond. 'A' on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of 'A'.
bond. 'A' on ozonolysis gives two moles of an aldehyde of molar mass 44 u. Write IUPAC name of 'A'.
Ans. As per the given information, ‘A' on ozonolysis gives two moles of an aldehyde of molar mass 44 u. The formation of two moles of an aldehyde indicates the presence of identical structural units on both sides of the double bond containing carbon atoms. Hence, the structure of ‘A' can be represented as:
XC = CX
There are eight C-H 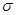 bonds. Hence, there are 8 hydrogen atoms in ‘A'. Also, there are three C-C bonds. Hence, there are four carbon atoms present in the structure of ‘A'.
bonds. Hence, there are 8 hydrogen atoms in ‘A'. Also, there are three C-C bonds. Hence, there are four carbon atoms present in the structure of ‘A'.
Combining the inferences, the structure of ‘A' can be represented as: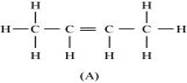
‘A' has 3 C-C bonds, 8 C-H 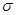 bonds, and one C-C π bond.
bonds, and one C-C π bond.
Hence, the IUPAC name of ‘A' is But-2-ene.
Ozonolysis of ‘A' takes place as: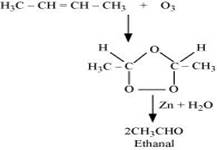
The final product is ethanal with molecular mass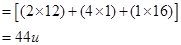
7. Propanal and pentan-3-one are the ozonolysis products of an alkene? What is the structural formula of the alkene?
Ans. As per the given information, propanal and pentan-3-one are the ozonolysis products of an alkene. Let the given alkene be ‘A'. Writing the reverse of the ozonolysis reaction, we get:
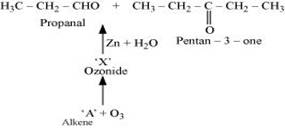
The products are obtained on the cleavage of ozonide ‘X'. Hence, ‘X' contains both products in the cyclic form. The possible structure of ozonide can be represented as:
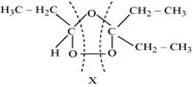
Now, ‘X' is an addition product of alkene ‘A' with ozone. Therefore, the possible structure of alkene ‘A' is:
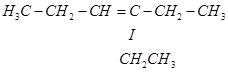
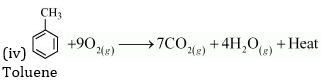
8. Write chemical equations for combustion reaction of the following hydrocarbons:
(i) Butane
(ii) Pentene
(iii) Hexyne
(iv) Toluene
Ans. Combustion can be defined as a reaction of a compound with oxygen.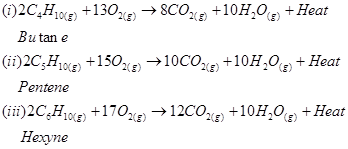
9. Draw the c is and trans structures of hex-2-ene. Which isomer will have higher b.p. and why?
Ans. Hex-2-ene is represented as:
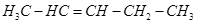
Geometrical isomers of hex-2-ene are:
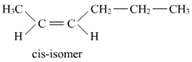
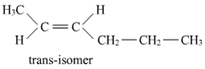
The dipole moment of c is-compound is a sum of the dipole moments of 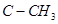 and
and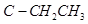 bonds acting in the same direction.
bonds acting in the same direction.
The dipole moment of trans-compound is the resultant of the dipole moments of 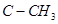 and
and 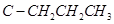 bonds acting in opposite directions.
bonds acting in opposite directions.
Hence, c is-isomer is more polar than trans-isomer. The higher the polarity, the greater is the intermolecular dipole-dipole interaction and the higher will be the boiling point. Hence, c is-isomer will have a higher boiling point than trans-isomer.
10. Why is benzene extra ordinarily stable though it contains three doublebonds?
Ans. Benzene is a hybrid of resonating structures given as:
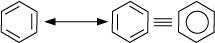
All six carbon atoms in benzene are 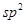 hybridized. The two
hybridized. The two 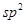 hybrid orbitals of each carbon atom overlap with the
hybrid orbitals of each carbon atom overlap with the 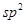 hybrid orbitals of adjacent carbon atoms to form six sigma bonds in the hexagonal plane. The remaining
hybrid orbitals of adjacent carbon atoms to form six sigma bonds in the hexagonal plane. The remaining 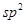 hybrid orbital on each carbon atom overlaps with the s-orbital of hydrogen to form six sigma C-H bonds. The remaining unhybridized p-orbital of carbon atoms has the possibility of forming three π bonds by the lateral overlap of
hybrid orbital on each carbon atom overlaps with the s-orbital of hydrogen to form six sigma C-H bonds. The remaining unhybridized p-orbital of carbon atoms has the possibility of forming three π bonds by the lateral overlap of .
.
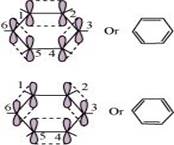
The six  s are delocalized and can move freely about the six carbon nuclei. Even after the presence of three double bonds, these delocalized
s are delocalized and can move freely about the six carbon nuclei. Even after the presence of three double bonds, these delocalized  -electrons stabilize benzene.
-electrons stabilize benzene.
11. What are the necessary conditions for any system to be aromatic?
Ans. A compound is said to be aromatic if it satisfies the following three conditions:
(i) It should have a planar structure.
(ii) The π-electrons of the compound are completely delocalized in the ring.
(iii) The total number of 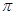 -electrons present in the ring should be equal to (4n + 2), where n = 0, 1, 2 … etc. This is known as Huckel's rule.
-electrons present in the ring should be equal to (4n + 2), where n = 0, 1, 2 … etc. This is known as Huckel's rule.
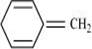
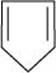
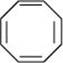
12. Explain why the following systems are not aromatic?
(i)
(ii)
(iii)
Ans. (i)
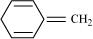
For the given compound, the number of  -electrons is six. But only four π-electrons are present within the ring. Also there is no conjugation of
-electrons is six. But only four π-electrons are present within the ring. Also there is no conjugation of  -electrons within the ring and the compound is not planar in shape. Hence, the given compound is not aromatic in nature.
-electrons within the ring and the compound is not planar in shape. Hence, the given compound is not aromatic in nature.
(ii)
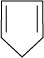
For the given compound, the number of 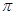 -electrons is four.
-electrons is four.
By Huckel's rule,
4n + 2 = 4
4n = 2
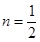
For a compound to be aromatic, the value of n must be an integer (n = 0, 1, 2…), which is not true for the given compound. Hence, it is not aromatic in nature.
(iii)
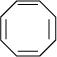
For the given compound, the number of 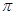 -electrons is eight.
-electrons is eight.
By Huckel's rule,
4n + 2 = 8
4n = 6
For a compound to be aromatic, the value of n must be an integer (n = 0, 1, 2…). Since the value of n is not an integer, the given compound is not aromatic in nature.
13. How will you convert benzene into
(i) p-nitrobromobenzene
(ii) m-nitrochlorobenzene
(iii) p -nitrotoluene
(iv) acetophenone
Ans. (i) Benzene can be converted into p-nitrobromobenzene as: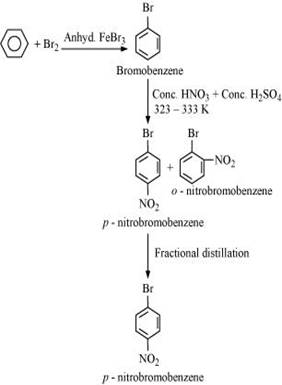
(ii) Benzene can be converted into m-nitrochlorobenzene as: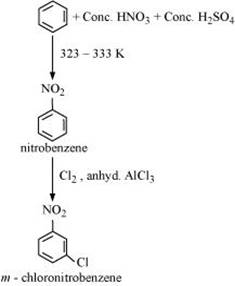
(iii) Benzene can be converted into p-nitrotoulene as: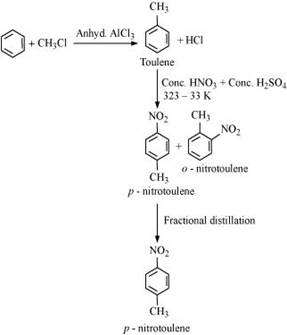
(iv) Benzene can be converted into acetophenone as: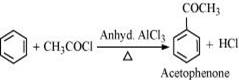
14.In the alkane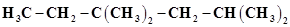 , identify 1°, 2°, 3° carbon atoms and give the number of H atoms bonded to each one of these.
, identify 1°, 2°, 3° carbon atoms and give the number of H atoms bonded to each one of these.
Ans.
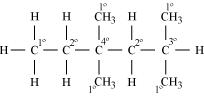
1° carbon atoms are those which are bonded to only one carbon atom, i.e., they have only one carbon atom as their neighbour. The given structure has five 1° carbon atoms and fifteen hydrogen atoms are attached to it.
2° carbon atoms are those which are bonded to two carbon atoms, i.e., they have two carbon atoms as their neighbours. The given structure has two 2° carbon atoms and four hydrogen atoms are attached to it.
3° carbon atoms are those which are bonded to three carbon atoms, i.e., they have three carbon atoms as their neighbours. The given structure has one 3° carbon atom and only one hydrogen atom is attached to it.
15.What effect does branching of an alkane chain has on its boiling point?
Ans. Alkanes experience inter-molecular Van der Waals forces. The stronger the force, the greater will be the boiling point of the alkane.
As branching increases, the surface area of the molecule decreases which results in a small area of contact. As a result, the Van der Waals force also decreases which can be overcome at a relatively lower temperature. Hence, the boiling point of an alkane chain decreases with an increase in branching.
16. Addition of HBr to propene yields 2-bromopropane, while in the presence of benzoyl peroxide, the same reaction yields 1-bromopropane. Explain and give mechanism.
Ans. Addition of HBr to propene is an example of an electrophilic substitution reaction.
Hydrogen bromide provides an electrophile,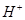 . This electrophile attacks the double bond to form 1° and 2° carbocations as shown:
. This electrophile attacks the double bond to form 1° and 2° carbocations as shown:
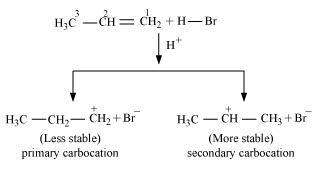
Secondary carbocations are more stable than primary carbocations. Hence, the former predominates since it will form at a faster rate. Thus, in the next step, Br- attacks the carbocation to form 2 - bromopropane as the major product.
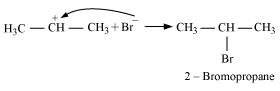
This reaction follows Markovnikov's rule where the negative part of the addendum is attached to the carbon atom having a lesser number of hydrogen atoms.
In the presence of benzoyl peroxide, an addition reaction takes place anti to Markovnikov's rule. The reaction follows a free radical chain mechanism as:

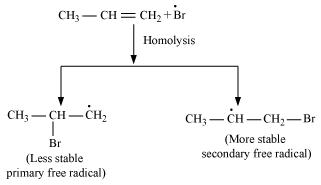
Secondary free radicals are more stable than primary radicals. Hence, the former predominates since it forms at a faster rate. Thus, 1 - bromopropane is obtained as the major product.
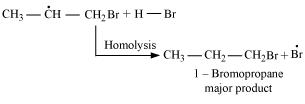
In the presence of peroxide, Br free radical acts as an electrophile. Hence, two different products are obtained on addition of HBr to propene in the absence and presence of peroxide.
17. Write down the products of ozonolysis of 1, 2-dimethylbenzene (o-xylene). How does the result support Kekule structure for benzene?
Ans. o-xylene has two resonance structures:
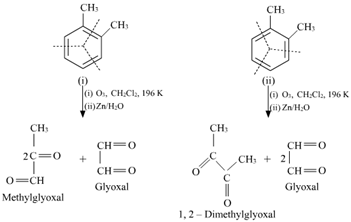
All three products, i.e., methyl glyoxal, 1, 2-dimethylglyoxal, and glyoxal are obtained from two Kekule structures. Since all three products cannot be obtained from any one of the two structures, this proves that o-xylene is a resonance hybrid of two Kekule structures (I and II).
18.Arrange benzene, n-hexane and ethyne in decreasing order of acidic behaviour. Also give reason for this behaviour.
Ans. Acidic character of a species is defined on the basis of ease with which it can lose its H-atoms.
The hybridization state of carbon in the given compound is:
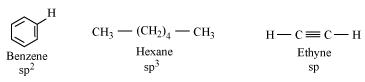
As the s-character increases, the electronegativity of carbon increases and the electrons of C-H bond pair lie closer to the carbon atom. As a result, partial positive charge of H-atom increases and 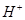 ions are set free.
ions are set free.
The s-character increases in the order:
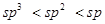 Hence, the decreasing order of acidic behaviour is Ethyne > Benzene > Hexane.
Hence, the decreasing order of acidic behaviour is Ethyne > Benzene > Hexane.
19. Why does benzene undergo electrophilic substitution reactions easily and nucleophilic substitutions with difficulty?
Ans. Benzene is a planar molecule having delocalized electrons above and below the plane of ring. Hence, it is electron-rich. As a result, it is highly attractive to electron deficient species i.e., electrophiles.
Therefore, it undergoes electrophilic substitution reactions very easily. Nucleophiles are electron-rich. Hence, they are repelled by benzene. Hence, benzene undergoes nucleophilic substitutions with difficulty.
20. How would you convert the following compounds into benzene?
(i) Ethyne
(ii) Ethene
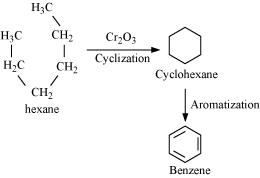
(iii) Hexane
Ans. (i) Benzene from Ethyne:

(ii) Benzene from Ethene:
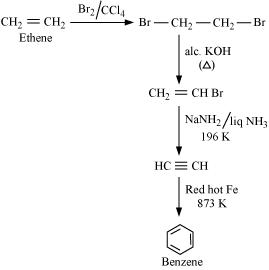
(iii) Hexane to Benzene
21. Write structures of all the alkenes which on hydrogenation give 2-methylbutane.
Ans. The basic skeleton of 2-methylbutane is shown below:
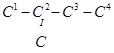
On the basis of this structure, various alkenes that will give 2-methylbutane on hydrogenation are:
(a)
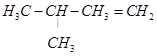
(b)
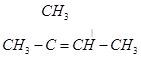
(c)
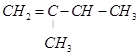
22. Arrange the following set of compounds in order of their decreasing relative reactivity with an electrophile, E+
(a) Chlorobenzene, 2,4-dinitrochlorobenzene, p-nitrochlorobenzene
(b) Toluene,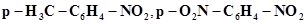 .
.
Ans. Electrophiles are reagents that participate in a reaction by accepting an electron pair in order to bond to nucleophiles.
The higher the electron density on a benzene ring, the more reactive is the compound towards an electrophile, E+ (Electrophilic reaction).
(a) The presence of an electron withdrawing group (i.e., 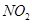 -and Cl-) deactivates the aromatic ring by decreasing the electron density.
-and Cl-) deactivates the aromatic ring by decreasing the electron density.
Since 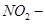 group is more electron withdrawing (due to resonance effect) than the Cl- group (due to inductive effect), the decreasing order of reactivity is as follows:
group is more electron withdrawing (due to resonance effect) than the Cl- group (due to inductive effect), the decreasing order of reactivity is as follows:
Chlorobenzene > p - nitrochlorobenzene > 2, 4 - dinitrochlorobenzene
(b) While 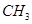 - is an electron donating group,
- is an electron donating group, 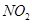 - group is electron withdrawing. Hence, toluene will have the maximum electron density and is most easily attacked by E+.
- group is electron withdrawing. Hence, toluene will have the maximum electron density and is most easily attacked by E+.
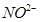 is an electron withdrawing group. Hence, when the number of
is an electron withdrawing group. Hence, when the number of 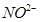 substituents is greater, the order is as follows:
substituents is greater, the order is as follows:
Toluene > 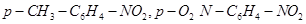
23. Out of benzene, m-dinitrobenzene and toluene which will undergo nitration most easily and why?
Ans. The ease of nitration depends on the presence of electron density on the compound to form nitrates. Nitration reactions are examples of electrophilic substitution reactions where an electron-rich species is attacked by a nitronium ion (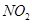 -).
-).
Now, 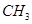 - group is electron donating and
- group is electron donating and 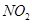 - is electron withdrawing. Therefore, toluene will have the maximum electron density among the three compounds followed by benzene. On the other hand, m- Dinitrobenzene will have the least electron density. Hence, it will undergo nitration with difficulty. Hence, the increasing order of nitration is as follows:
- is electron withdrawing. Therefore, toluene will have the maximum electron density among the three compounds followed by benzene. On the other hand, m- Dinitrobenzene will have the least electron density. Hence, it will undergo nitration with difficulty. Hence, the increasing order of nitration is as follows:
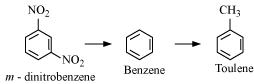
24. Suggest the name of a Lewis acid other than anhydrous aluminium chloride which can be used during ethylation of benzene.
Ans. The ethylation reaction of benzene involves the addition of an ethyl group on the benzene ring. Such a reaction is called a Friedel-Craft alkylation reaction. This reaction takes place in the presence of a Lewis acid.
Any Lewis acid like anhydrous 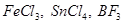 etc. can be used during the ethylation of benzene.
etc. can be used during the ethylation of benzene.
25. Why is Wurtz reaction not preferred for the preparation of alkanes containing odd number of carbon atoms? Illustrate your answer by taking one example.
Ans. Wurtz reaction is limited for the synthesis of symmetrical alkanes (alkanes with an even number of carbon atoms) In the reaction, two similar alkyl halides are taken as reactants and an alkane, containing double the number of carbon atoms, are formed. Example: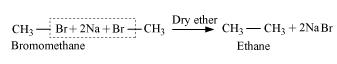
Wurtz reaction cannot be used for the preparation of unsymmetrical alkanes because if two dissimilar alkyl halides are taken as the reactants, then a mixture of alkanes is obtained as the products. Since the reaction involves free radical species, a side reaction also occurs to produce an alkene. For example, the reaction of bromomethane and iodoethane gives a mixture of alkanes.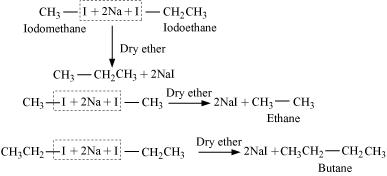
The boiling points of alkanes (obtained in the mixture) are very close. Hence, it becomes difficult to separate them.
