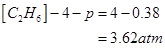Equilibrium - Solutions
CBSE Class 11 Chemistry
NCERT Solutions
Chapter 7
EQUILIBRIUM
1. A liquid is in equilibrium with its vapour in a sealed container at a fixed temperature. The volume of the container is suddenly increased.
a) What is the initial effect of the change on vapour pressure?
b) How do rates of evaporation and condensation change initially?
c) What happens when equilibrium is restored finally and what will be the final vapour pressure?
Ans. (a) If the volume of the container is suddenly increased, then the vapour pressure would decrease initially. This is because the amount of vapour remains the same, but the volume increases suddenly. As a result, the same amount of vapour is distributed in a larger volume.
(b) Since the temperature is constant, the rate of evaporation also remains constant. When the volume of the container is increased, the density of the vapour phase decreases. As a result, the rate of collisions of the vapour particles also decreases. Hence, the rate of condensation decreases initially.
(c) When equilibrium is restored finally, the rate of evaporation becomes equal to the rate of condensation. In this case, only the volume changes while the temperature remains constant. The vapour pressure depends on temperature and not on volume. Hence, the final vapour pressure will be equal to the original vapour pressure of the system.
2. What is 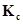 for the following equilibrium when the equilibrium concentration of each substance is: [
for the following equilibrium when the equilibrium concentration of each substance is: [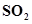 ]= 0.60 M, [
]= 0.60 M, [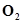 ] = 0.82 M and [
] = 0.82 M and [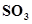 ] = 1.90 M ?
] = 1.90 M ? 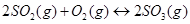
Ans. The equilibrium constant (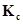 ) for the give reaction is:
) for the give reaction is:
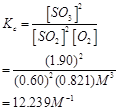
Hence, Kc for the equilibrium is 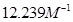 .
.
3. At a certain temperature and total pressure of 105 Pa, iodine vapour contains 40% by volume of I atoms 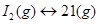 Calculate
Calculate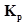 for the equilibrium.
for the equilibrium.
Ans. Partial pressure of I atoms,
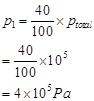
Partial pressure of 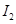 molecules,
molecules,
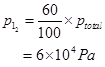
Now, for the given reaction,
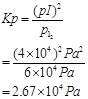
4. Write the expression for the equilibrium constant, Kc for each of the following reactions:
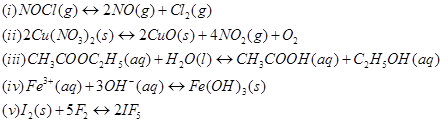
Ans.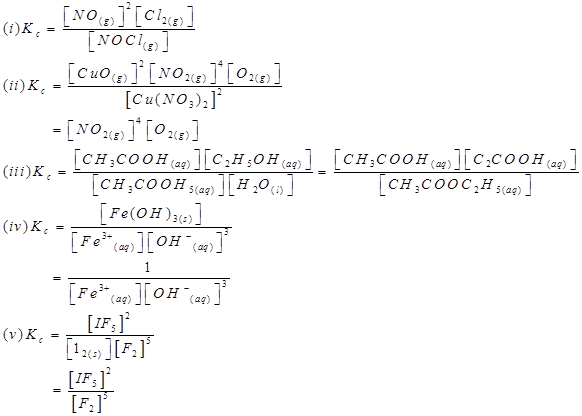
5. Find out the value of Kc for each of the following equilibria from the value of Kp:
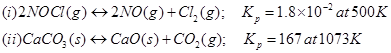
Ans. The relation between 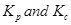 is given as:
is given as:
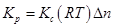
(i) Here,
Δn = 3 - 2 = 1
R = 0.0831 bar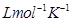
T = 500 K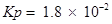
Now,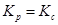 (RT) Δn
(RT) Δn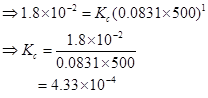
(ii) Here,
Δn = 2 - 1 = 1
R = 0.0831 bar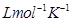
T = 1073 K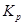 = 167
= 167
Now,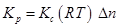
6. For the following equilibrium, 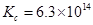
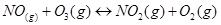 Both the forward and reverse reactions in the equilibrium are elementary bimolecular reactions. What is
Both the forward and reverse reactions in the equilibrium are elementary bimolecular reactions. What is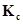 , for the reverse reaction?
, for the reverse reaction?
Ans. It is given that 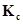 for the forward reaction is
for the forward reaction is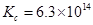
As we know that from the given information, the rate of backward reaction is the reciprocal of the rate of forward reaction:
Then, 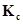 for the reverse reaction will be
for the reverse reaction will be
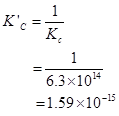
7. Explain why pure liquids and solids can be ignored while writing the equilibrium constant expression?
Ans. For a pure substance (both solids and liquids),

Now, the molecular mass and density (at a particular temperature) of a pure substance is always fixed and is accounted for in the equilibrium constant. Therefore, the values of pure substances are not mentioned in the equilibrium constant expression.
8. Reaction between 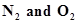 takes place as follows:
takes place as follows: 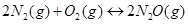
If a mixture of 0.482 mol of 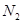 and 0.933 mol of
and 0.933 mol of 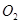 is placed in a 10 L reaction vessel and allowed to form
is placed in a 10 L reaction vessel and allowed to form 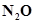 at a temperature for which
at a temperature for which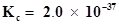 , determine the composition of equilibrium mixture.
, determine the composition of equilibrium mixture.
Ans. Let the concentration of 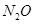 at equilibrium be x.
at equilibrium be x.
The given reaction is:
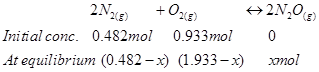
Therefore, at equilibrium, in the 10 L vessel:
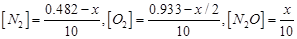
The value of equilibrium constant i.e., 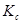
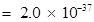 is very small. Therefore, the amount of
is very small. Therefore, the amount of 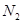 and
and 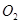 reacted is also very small. Thus, x can be neglected from the expressions of molar concentrations of
reacted is also very small. Thus, x can be neglected from the expressions of molar concentrations of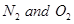 .
.
Then,
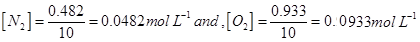
Now,
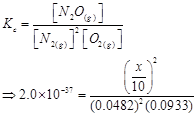
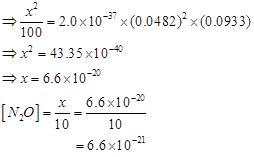
9. Nitric oxide reacts with 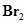 and gives nitrosyl bromide as per reaction given below:
and gives nitrosyl bromide as per reaction given below:
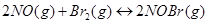
When 0.087 mol of NO and 0.0437 mol of 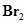 are mixed in a closed container at constant temperature, 0.0518 mol of NO Br is obtained at equilibrium. Calculate equilibrium amount of NO and
are mixed in a closed container at constant temperature, 0.0518 mol of NO Br is obtained at equilibrium. Calculate equilibrium amount of NO and 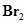 .
.
Ans. The given reaction is:
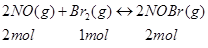
Now, 2 mol of NOBr are formed from 2 mol of NO. Therefore, 0.0518 mol of NOBr are formed from 0.0518 mol of NO.
Again, 2 mol of NOBr are formed from 1 mol of Br.
Therefore, 0.0518 mol of NOBr are formed from mol of Br, or 0.0259 mol of NO.
The amount of NO and Br present initially is as follows:
[NO] = 0.087 mol [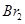 ] = 0.0437 mol
] = 0.0437 mol
Therefore, the amount of NO present at equilibrium is:
[NO] = 0.087 - 0.0518
= 0.0352 mol
And, the amount of Br present at equilibrium is:
[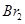 ] = 0.0437 - 0.0259
] = 0.0437 - 0.0259
= 0.0178 mol
10. At 450 K, 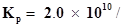 bar for the given reaction at equilibrium.
bar for the given reaction at equilibrium.
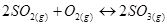
What is 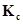 at this temperature?
at this temperature?
Ans. For the given reaction,![]()
T = 450 K
R = 0.0831 bar L bar 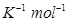
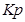 =
= 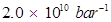
We know that,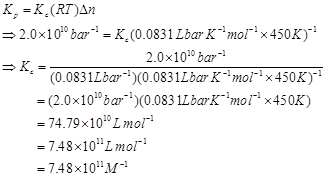
11. A sample of 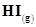 is placed in flask at a pressure of 0.2 atm. At equilibrium the partial pressure of HI(g) is 0.04 atm. What is
is placed in flask at a pressure of 0.2 atm. At equilibrium the partial pressure of HI(g) is 0.04 atm. What is 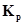 for the given equilibrium?
for the given equilibrium?
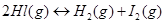
Ans. The initial concentration of HI is 0.2 atm. At equilibrium, it has a partial pressure of 0.04 atm. Therefore, a decrease in the pressure of HI is 0.2 - 0.04 = 0.16. The given reaction is: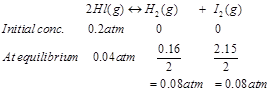
Therefore,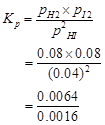
Hence, the value of 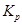 for the given equilibrium is 4.0.
for the given equilibrium is 4.0.
12. A mixture of 1.57 mol of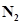 , 1.92 mol of
, 1.92 mol of 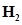 and 8.13 mol of
and 8.13 mol of 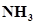 is introduced into a 20 L reaction vessel at 500 K. At this temperature, the equilibrium constant,
is introduced into a 20 L reaction vessel at 500 K. At this temperature, the equilibrium constant, 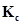 for the reaction
for the reaction 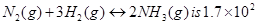
Is the reaction mixture at equilibrium? If not, what is the direction of the net reaction?
Ans. The given reaction is: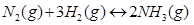
The given concentrations of various species is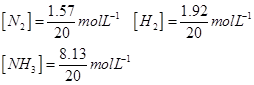
Now, reaction quotient 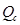 is:
is: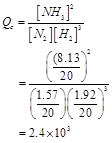
Since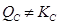 , the reaction mixture is not at equilibrium.
, the reaction mixture is not at equilibrium.
Again, 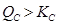 . Hence, the reaction will proceed in the reverse direction.
. Hence, the reaction will proceed in the reverse direction.
13. The equilibrium constant expression for a gas reaction is,
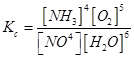
Write the balanced chemical equation corresponding to this expression.
Ans. The balanced chemical equation corresponding to the given expression can be written as: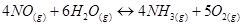
14. One mole of 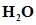 and one mole of CO are taken in 10 L vessel and heated to
and one mole of CO are taken in 10 L vessel and heated to
725 K. At equilibrium 40% of water (by mass) reacts with CO according to the equation,
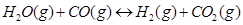
Calculate the equilibrium constant for the reaction.
Ans. The given reaction is: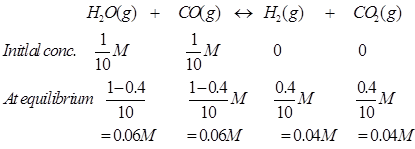
Therefore, the equilibrium constant for the reaction,
We know that: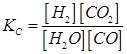
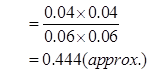
15. At 700 K, equilibrium constant for the reaction 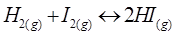 is 54.8. If 0.5
is 54.8. If 0.5 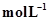 of
of 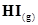 is present at equilibrium at 700 K, what are the concentration of
is present at equilibrium at 700 K, what are the concentration of 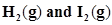 assuming that we initially started with
assuming that we initially started with 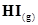 and allowed it to reach equilibrium at 700 K?
and allowed it to reach equilibrium at 700 K?
Ans. It is given that equilibrium constant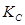 for the reaction
for the reaction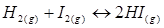 is 54.8.
is 54.8.
Therefore, at equilibrium, the equilibrium constant 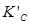 for the reaction
for the reaction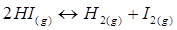 will be
will be 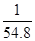 .
.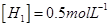
Let the concentrations of hydrogen and iodine at equilibrium be x 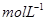
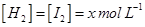 .
.
Therefore,
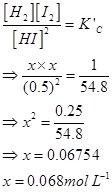
Hence, at equilibrium,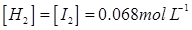
16. What is the equilibrium concentration of each of the substances in the equilibrium when the initial concentration of ICl was 0.78 M?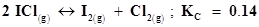
Ans. The given reaction is: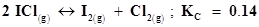

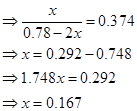
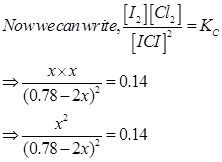
Hence, at equilibrium,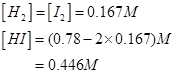
17. 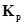 = 0.04 atm at 899 K for the equilibrium shown below. What is the equilibrium concentration of
= 0.04 atm at 899 K for the equilibrium shown below. What is the equilibrium concentration of 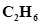 when it is placed in a flask at 4.0 atm pressure and allowed to come to equilibrium?
when it is placed in a flask at 4.0 atm pressure and allowed to come to equilibrium?
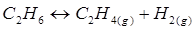
Ans. Let p be the pressure exerted by ethene and hydrogen gas (each) at equilibrium.
Now, according to the reaction,
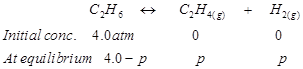
We can write,
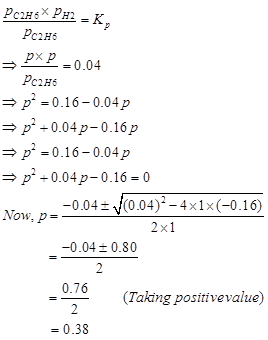
Hence, at equilibrium,