Chemical Effects of Electric Current - Exemplar Solutions 3
NCERT Exemplar Solutions
CHAPTER – 14
Chemical Effects of Electric Current
SHORT ANSWER QUESTIONS
12. Boojho made the circuit given in Fig. 14.3 and observed that the bulb did not glow. On Paheli’s suggestion, he added one more cell in the circuit. The bulb now glows. Explain.
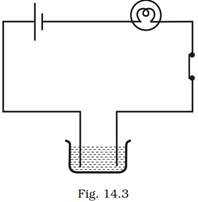
Ans: Addition of another cell in the circuit increased the current flowing through the bulb. The increased current was sufficient to make the bulb glow.
13. Paheli set up an experiment using liquid A in the beaker as shown in Fig. 14.4. She observed that the bulb glows. Then she replaced the liquid A by another liquid B. This time the bulb did not glow. Boojho suggested replacing the bulb by an LED. They observed that the LED glows. Explain.
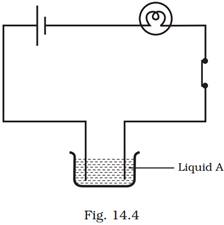
Ans: The current through liquid B could be weak and therefore unable to make the bulb glow. However, it was strong enough for the LED to glow. LED needs lesser current than that required by a bulb.
14. Paheli wants to deposit silver on an iron spoon. She took silver nitrate (AgNO3) solution in a beaker and setup a simple circuit for electroplating. Which terminal of the battery should the spoon be connected to? What material should the other electrode be made of?
Ans: The iron spoon should be connected to the negative terminal (cathode) of the battery. On passing the current, silver nitrate dissociates into silver ions and nitrate ions. Silver ions get drawn to the electrode connected to negative terminal of the battery and get deposited on it. To restore the loss of silver ions from the silver nitrate solution, the other electrode connected to the positive terminal of the battery should be made of silver. An equal amount of silver from the anode will dissolve into the solution and the process of electroplating will continue.
15. Why is tin electroplated on iron to make cans used for storing food?
Ans: Tin is less reactive than iron. Tin coating on iron prevents food from coming in contact with iron can and thus prevents it from getting spoiled.
16. Observe Fig. 14.5.
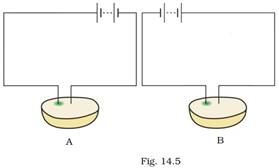
Which of these two circuits A or B shows the correct observation?
Ans: Diagram A shows the correct observation.
Explanation: Circuit A shows the correct observation because in this circuit, wire connected to the positive terminal of the battery has a greenish blue spot.
17. Observe the following circuits carefully. In which circuit will the bulb glow. Write ‘Yes’ or ‘No’ in the blank space provided along each of the circuit given in Fig. 14.6.
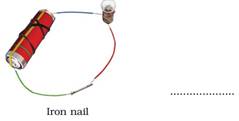
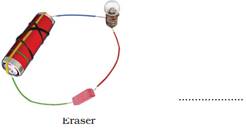
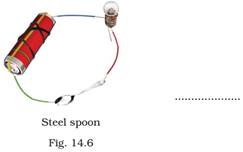
Ans: (a) No
(b) Yes. Iron is a metal and hence a good conductor of electricity.
(c) No. The material of an eraser is not a conductor of electricity.
(d) Yes. Steel is an alloy of metals and hence a good conductor of electricity.
