Chemical Bonding and Molecular Structure - Test Papers
CBSE TEST PAPER-01
CLASS - XI CHEMISTRY (Chemical Bonding and Molecular Structure)
General Instruction:
- All questions are compulsory.
- Marks are given alongwith their questions.
1. Define a chemical bond.
2. Give the main feature of Lewis approach of chemical bonding.
3. Write electron dot structure (Lewis structure) of Na, Ca, B, Br, Xe, As, Ge, N3-.
6. Define electrovalent bond.
7. Give the octet rule in short.
4. Give the main feature of Kossel’s explanation of chemical bonding.
5. How can you explain the formation of NaCl according to kossel concept?
8. Write the significance of octet rule.
9. Write the Lewis structure for CO molecule
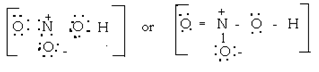
10. Give the Lewis dot structure of HNO3.
CBSE TEST PAPER-01
CLASS - XI CHEMISTRY (Chemical Bonding and Molecular Structure)
[Answers]
Ans 1. The attractive force which holds various constituents (atoms, ions etc.) together in different chemical species is called a chemical bond.
Ans 2. Lewis postulated that atoms achieve the stable octet when they are linked by chemical bonds. He assured that atoms are positively charged centre and the outer shell that could accommodate a maximum of eight electrons. These electrons occupy the corners of a cube which surrounds the centre. Lewis introduced simple notations to represent valence electrons in an atom called Lewis symbol.
Ans 3.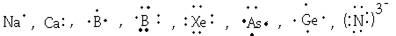
Ans 4. The bond formed, as a result of the electrostatic attraction between the positive and negative ions are termed as the electrovalent bond.
Ans 5. The atoms tend to adjust the arrangement of their electrons in such a way that they (except H and He) achieve eight electrons in their outermost shell. This is known as the octet rule.
Ans 6. Kossel in relation to chemical bonding drew attention to the following facts –
i) In the periodic table, the highly electronegative halogens and the highly electropositive alkali metals are separated by the noble gases.
ii) In the formation of a negative ion from a halogen atom and a positive ion from an alkali metal, atom is associated with a gain and loss of an electron by the respective atoms.
iii) The negative and positive ions so formed attain stable noble gas electronic configurations. The noble gases have particularly eight electrons, ns2 np6.
iv) The –ve and +ve ions are stabilized by electrostatic attraction.
Ans 7. The formation of NaCl from sodium and chlorine can be explained as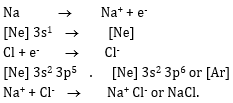
Ans 8. Octet rule signifies –
i) It is useful for understanding the structures of most of the organic compounds.
ii) It mainly applies to the second period elements of the periodic table.
Ans 9. (i) The outer (valence) shell configurations of carbon and oxygen atoms are
Carbon : (6) – 1s2 2s2 2p2
Oxygen : (8) – 1s2 2s2 2p4.
The valence electrons (4 + 6 = 10)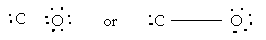
But it does not complete octet, thus multiple bond is exhibited.
Thus, 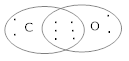

(ii) N (2s2 2p3), O (2s2 2p4)
5 + (2 x 6) + 1 = 18 electrons.
Thus,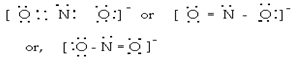
Ans 10. HNO3 ®